Asymptomatic localized pleural amyloidosis mimicking malignant pleural mesothelioma: report of a case
Introduction
Amyloidosis is characterized by the extracellular deposition of an amyloid substance (1), and it is classified into systemic and localized types. In primary systemic amyloidosis, peripheral nerves, heart, skin, muscle, and less frequently, the pulmonary tract may be involved. Although the presence of amyloid deposits in the lung parenchyma has been sometimes reported in the literature, pleural amyloidosis is extremely rare (2).
Case presentation
A pleural thickness was found on a routine chest X-ray of an asymptomatic 65-year-old man. He had no previous medical history. He had 80 pack-year smoking habit. His occupation was in the oil stove repair, and he had history of exposure to asbestos.
His physical examinations revealed that all values were within the normal limits, pulmonary function tests were within the normal limits. Laboratory examinations revealed: complete blood count and serological data including the C-reactive protein level remained within the normal limits. His serum levels of carcinoembryonic antigen (CEA) and cytokeratin 19 fragment (CYFRA) were 4.8 ng/mL (normal range, 0−5 ng/mL) and 2.5 U/mL (normal range, 0−3.5 U/mL), respectively.
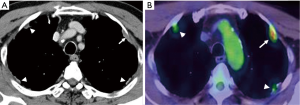
Chest computed tomography (CT) revealed that the pleural thickness, plaque, and tumor were diffusely located in the bilateral thoracic cavity (Figure 1A). The intrapulmonary tumor was not found. 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) showed that the maximum standardized uptake value (SUVmax) of this left sided pleural tumor was 4.84, and other lesions revealed a lower uptake (Figure 1B), and therefore malignancy could be suspected. No hilar and mediastinal lymphadenopathy, distant metastasis, or abnormal findings of other organs were found CT and positron emission tomography-computed tomography (PET-CT). The thoracotomy surgery was performed for the purpose of diagnosis and local disease control.
A left posterolateral thoracotomy at the fifth intercostal space was performed, Partial visceral pleurectomy including the mass was carried, whereas the mass were not found on the parietal pleura of the chest wall. The visceral pleurectomy for the left upper lobe of the lung, where two masses were palpated on the visceral pleural surface was completed (Figure 2) (3). Intraoperative frozen-section analysis of the specimen showed no malignancy. The other tumor was present on the left S6 and removed by lung parenchyma wedge resection using a stapler. Defect visceral pleura was repaired with polyglycolic acid sheet and fibrin glue. Prolonged pulmonary air leakage was not found, and postoperative course was uneventful.

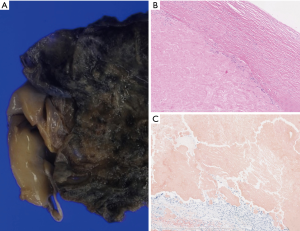
The cut surface of pleural mass revealed a hyalinizing structure and fibrillary thickened pleura with calcification (Figure 3A). Histopathologically, the lesion mainly comprised amyloid deposition showing Congo red stain was positive in the pleural tissue, chronic inflammatory cell infiltrate and multinucleated giant cell were found (Figure 3B,C). The stainability of Congo red stain was not lost by potassium permanganate treatment, the lesion was pathologically confirmed to be amyloidosis, excluding the amyloid protein A (AA) type. The appearance of serum monoclonal protein (M protein) was not found, there are no significant clinical symptom of multiple myeloma. Therefore, primary immunoglobulin light chain (AL) type amyloidosis of pleura was suggested. No symptoms of other organ amyloidosis have occurred 1 year after surgery.
Discussion
Amyloidosis is characterized by the deposition of an amyloid substance (1). There are four major categories primary or immunoglobulin AL disease, secondary or AA disease, hereditary or mutant transthyretin (ATTR) disease, and dialysis-associated or β2-microglobulin (β2M) disease (4). AL disease arises from the deposition of monoclonal κ or λ immunoglobulin light chain, in these cases, there are two sub-type of disease: systemic and localized. Amyloid is deposited in several organs and leads to organs failure in systemic disease. In this case, clinical course, family history, blood test and pathological examination showed primary localized pleural AL type amyloidosis.
Berk et al. (4) reported that localized amyloid deposits arise from a small number of plasma cells surrounding the lesion, and the most commonly involved sites were the skin, urethra, and urinary bladder, eye and lung parenchyma. Localized pulmonary amyloidosis has been reported on many occasions (4), but pleural amyloidosis is rare (2). The cases of pleural amyloidosis accompanied with pleural effusion was previously reported (5,6), they had symptom of dyspnea etc. Therefore asymptomatic localized pleural amyloidosis like this patient is extremely rare. Adams et al. reported that the most important diagnosis of pleural amyloid includes mesothelioma, either a sarcomatoid or the unusual desmoplastic variant, and solitary fibrous tumor of the pleura (5).
The case with the detection of a pulmonary amyloid lesion by FDG-PET was reported, the uptake of FDG could be related to the abnormal production of immunoglobulins by plasma cells (7). This false-positive findings of PET-CT makes it difficult to diagnose as pleural amyloidosis in distinguishing malignancy.
In conclusion, we experienced a case of localized pleural amyloidosis mimicking pleural mesothelioma clinically and radiologically. In the future, the detection of pleural lesion is supposed to increase with prevalence of CT screening. And false-negative diseases like as this case outside of malignant pleural dissemination and pleural mesothelioma may be increasing by prevalence of PET-CT. Localized pleural amyloidosis should be considered as one of the differential diagnosis for a pleural lesion.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Glenner GG. Amyloid deposits and amyloidosis. N Engl J Med 1980;302:1283-92,1333-43. [PubMed]
- Bontemps F, Tillie-Leblond I, Coppin MC, et al. Pleural amyloidosis: thoracoscopic aspects. Eur Respir J 1995;8:1025-7. [PubMed]
- Nakano T, Endo S, Tetsuka K, et al. Thoracotomy findings (top, lateral side; left, cranial side). Asvide 2016;3:057. Available online: http://www.asvide.com/articles/808
- Berk JL, O'Regan A, Skinner M. Pulmonary and tracheobronchial amyloidosis. Semin Respir Crit Care Med 2002;23:155-65. [PubMed]
- Adams AL, Castro CY, Singh SP, et al. Pleural amyloidosis mimicking mesothelioma: a clinicopathologic study of two cases. Ann Diagn Pathol 2001;5:229-32. [PubMed]
- Maeno T, Sando Y, Tsukagoshi M, et al. Pleural amyloidosis in a patient with intractable pleural effusion and multiple myeloma. Respirology 2000;5:79-80. [PubMed]
- Kung J, Zhuang H, Yu JQ, et al. Intense fluorodeoxyglucose activity in pulmonary amyloid lesions on positron emission tomography. Clin Nucl Med 2003;28:975-6. [PubMed]


