
Airway interventions for tracheobronchial involvement in esophageal carcinoma: a retrospective cohort outcome study and algorithmic approach
Introduction
Airway involvement may be encountered in esophageal carcinoma (EC) due to proximity of the tracheobronchial tree with the esophagus (1,2). Tracheobronchoesophageal fistulas (TEF) develop in 5–15% of EC patients (2-4), and contribute to increased morbidity and mortality due to recurrent aspiration and pneumonia (5,6). The majority of symptomatic patients present with unresectable advanced disease. As such, management is often centered on palliation of symptoms including dyspnea, pain, and dysphagia, where esophageal stenting has been established as a treatment for dysphagia (3,7-10). Airway interventions including balloon or rigid bronchoscopic barrel dilatation, stenting, thermal photo ablation and tracheostomy have been used in the treatment of airway involvement in EC. However, there is a paucity of literature on airway interventions in EC, with current data originating mostly from small series, which may include primary malignancies other than EC. There is a wide range of survival rates following airway interventions in EC reported in the literature, ranging from 0.8 to 7.1 months (7,11-15). There is also limited data on risk factors for TEF formation and survival in EC patients with airway involvement to guide clinical decision making (6,16,17). This study aims to evaluate risk factors for TEF formation and survival in patients with EC who require airway interventions, and proposes an algorithmic management approach based on the findings and a review of literature. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-138/rc).
Methods
Electronic medical records were assessed to identify patients diagnosed with EC between 1998 and 2018 at a tertiary medical centre in Singapore. Consecutive patients with EC and also requiring airway intervention for tracheobronchial involvement were included in a retrospective analysis. All patients had bronchoscopic airway evaluation. Patients without bronchoscopic airway evaluation were excluded. Demographics, clinical progress, treatment and survival outcomes were recorded. Staging at the time of diagnosis was determined based on clinical, or pathological staging, if available. A positive change in either patient reported respiratory symptoms such as dyspnea, cough and hemoptysis, or objective measures such as liberation from mechanical ventilation, reduction in oxygen requirements or improvement in oxygen saturation, were used to define improvement in respiratory symptoms. Patients were followed up till death or until the completion of the study. This study presents additional information on a subset of patients whose data had been previously published (2), analysing data specifically in patients who required airway intervention only. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of SingHealth CIRB C (approval number: 2017-2966). Requirement for informed consent was waived, as the study was retrospective in nature and data was de-identified prior to analysis.
Airway interventions
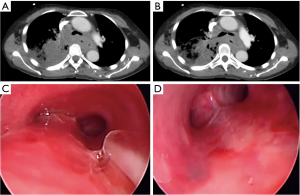
Airway involvement by EC was diagnosed bronchoscopically and included mucosal invasion (Figure 1), extrinsic compression with airway luminal diameter reduced by at least 20% for a length of ≥20 mm (Figure 2), or TEF (Figure 3) (2,18). Malignant tracheobronchial strictures were dilated using controlled radial expansion (CRE) balloons (Microinvasive, Boston Scientific Corporation, Massachusetts, USA) or rigid bronchoscope barrel. Neodymium-doped yttrium aluminium garnet (Nd-YAG) laser (LaserSonics, Milpitas, California, USA) and argon plasma coagulation (Ceralas®, Biolitec, Jena, Germany) were used for tumour ablation. Tracheostomy was performed for proximal tracheal stenosis. When there was extrinsic compression or luminal obstruction by EC, or presence of TEF, covered self-expanding metallic stents (SEMS) (UltraflexTM, Boston Scientific Corporation, Massachusetts, USA), or silicone straight or Y-stents (TracheobronxaneTM Dumon, Novatech, La Ciotat, France) were deployed according to the operator’s clinical judgement (Videos 1,2) (18).
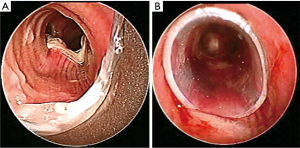
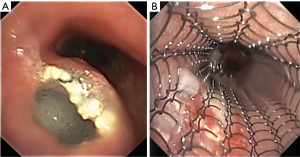
Anatomical relationship of esophagus to tracheobronchial tree
The upper esophagus [located 15 to 25 centimeters (cm) from upper incisors] comprises of the cervical and thoracic esophagus till the level of the inferior border of the azygos vein. The posterior membranous wall of the trachea is anterior to the esophagus, up to the tracheal bifurcation at the main carina. The mid esophagus (25 to 30 cm from upper incisors) spans the tracheal bifurcation to the proximal left main bronchus, while the lower esophagus (30 to 40 cm from upper incisors) extends from distal left main bronchus to left lower lobe bronchus (19,20). Gastroesophageal junction carcinoma rarely causes direct invasion of the tracheobronchial tree because of lack of proximity, and were excluded from analysis.
Statistical analysis
Continuous variables, presented as mean ± standard deviation (SD) or as median (interquartile range, IQR), were analysed using t-tests or Mann-Whitney U tests as appropriate. Discrete variables were analysed using Pearson’s chi-square or Fisher’s exact test. Cox proportional hazard regression was used to explore associations between survival, patient characteristics, anatomical involvement as well as treatment modalities.
For hazard ratio analysis, survival period was defined as the time from airway intervention to death or till completion of study. The Kaplan-Meier method and log-rank test were used to compare the overall survival of patients with and without TEF at time of airway intervention. Statistical differences were considered significant at P<0.05. Statistical analyses were performed using SPSS (SPSS, version 17.0; SPSS Inc., Chicago, IL, USA).
Results
Demographics, airway involvement and treatment (Table 1)
Table 1
| Characteristics | Number (%) | Upper esophageal carcinoma (n=33), (%) | Mid esophageal carcinoma (n=78), (%) | Lower esophageal carcinoma (n=11), (%) | P value |
|---|---|---|---|---|---|
| Patient demographics and airway involvement | |||||
| Mean age at diagnosis in years ± SD | 61±11 | 59±12 | 64±10 | 67±9 | 0.114 |
| Male gender | 109 (89.3) | 29 (78.8) | 72 (92.3) | 8 (72.7) | 0.136 |
| Chinese ethnicity | 110 (90.2) | 29 (78.8) | 73 (93.6) | 8 (72.7) | 0.082 |
| Squamous cell carcinoma | 116 (95.1) | 32 (97.0) | 76 (97.4) | 7 (63.6) | <0.001 |
| Stage 3 or 4 at time of diagnosis | 98 (80.3) | 30 (90.9) | 64 (82.1) | 4 (36.4) | <0.001 |
| Type of airway involvement | |||||
| Mucosal invasion | 24 (19.7) | 8 (24.2) | 13 (16.7) | 3 (27.3) | 0.526 |
| Extrinsic compression ≥20% | 7 (5.7) | 4 (12.1) | 3 (3.8) | 0 (0) | 0.159 |
| Mucosal invasion and extrinsic compression ≥20% | 47 (38.5) | 11(33.3) | 30 (38.5) | 6 (54.5) | 0.457 |
| Tracheo-esophageal fistula | 44 (36.1) | 10 (30.3) | 32 (41.0) | 2 (18.2) | 0.243 |
| Primary site of airway involvement* | |||||
| Proximal trachea | 23 (18.9) | 13 (39.4) | 8 (10.3) | 2 (18.2) | 0.002 |
| Mid trachea | 31 (25.4) | 14 (42.4) | 15 (19.2) | 2 (18.2) | 0.039 |
| Distal trachea | 18 (14.8) | 3 (9.1) | 15 (19.2) | 0 (0.0) | 0.136 |
| Carina | 3 (2.5) | 0 (0.0) | 3 (3.8) | 0 (0.0) | 0.420 |
| Left main bronchus | 41 (33.6) | 2 (6.1) | 35 (44.9) | 4 (36.4) | <0.001 |
| Others** | 6 (4.9) | 1 (3.0) | 2 (2.5) | 3 (27.2) | 0.011 |
| Airway lesions ≤20 mm from or at the carina | 73 (59.8) | 10 (30.3) | 57 (73.1) | 6 (54.5) | <0.001 |
| Airway interventions | |||||
| Airway stenting | 100 (82.0) | 22 (66.7) | 70 (89.7) | 8 (72.7) | 0.011 |
| Tracheostomy | 15 (12.3) | 10 (30.3) | 5 (6.4) | 0 (0.0) | <0.001 |
| Treatment received prior to airway intervention | |||||
| Radiotherapy | 44 (36.1) | 13 (4.3) | 26 (33.3) | 5 (45.5) | 0.660 |
| Chemotherapy | 52 (42.6) | 13 (4.3) | 31 (39.7) | 8 (72.7) | 0.106 |
| Radiotherapy and chemotherapy | 33 (27.0) | 8 (24.2) | 21 (26.9) | 4 (36.4) | 0.735 |
| Esophageal stenting | 17 (13.9) | 2 (6.1) | 13 (16.7) | 2 (18.2) | 0.308 |
| Surgery | 22 (18.0) | 3 (9.1) | 13 (16.7) | 6 (54.5) | 0.003 |
| Treatment received during entire course of disease | |||||
| Radiotherapy | 96 (78.7) | 30 (90.9) | 59 (75.6) | 7 (63.6) | 0.088 |
| Chemotherapy | 78 (63.9) | 22 (66.7) | 48 (61.5) | 8 (72.7) | 0.715 |
| Radiotherapy and chemotherapy | 68 (55.7) | 21 (63.6) | 41 (52.6) | 6 (54.5) | 0.560 |
| Esophageal stenting | 46 (37.7) | 5 (15.1) | 38 (48.7) | 3 (27.3) | 0.003 |
| Surgery | 25 (20.5) | 6 (18.2) | 13 (16.7) | 6 (54.5) | 0.013 |
*, data unavailable for 2 patients; **, right main bronchus, left lower lobe, right bronchus intermedius, right middle lobe, left upper lobe, right upper lobe, right lower lobe, apical segment of right lower lobe. SD, standard deviation.
There were 122 patients recruited (Table 1). Squamous cell carcinoma was the histologic subtype in 95.1%. Combined airway mucosal invasion and extrinsic compression (47/122, 38.5%) was the most common bronchoscopic finding followed by TEF (44/122, 36.1%). The left main bronchus was the most common airway site for mid EC (n=78), affecting 35/78, 44.9%. Fifty-seven out of 78, 73.1% of mid EC tumours were within 20mm of the carina.
Airway stenting was performed in 100 patients (100/122, 82%). Overall, 15 patients (15/122, 12.3%) underwent tracheostomy to manage airway obstruction. Patients with mid EC required airway stenting in 70/78, 89.7%, compared to lower (8/11, 72.7%) and upper EC lesions (22/33, 66.7%; p=0.011). Esophageal stenting was performed in 46/122, 37.7%. Patients with mid EC lesions required esophageal stenting procedures in 38/78, 48.7%, as compared to lower EC (3/11, 27.3%) and upper EC (5/33, 15.1%; P=0.003).
Types, complications and outcomes of airway intervention (Table 2)
Table 2
| Airway intervention modalities | n (%) unless specified |
|---|---|
| Tracheostomy | 15 (12.3) |
| Airway stenting | 100 (82.0) |
| Straight silicone stent | 54 (54.0) |
| Y silicone stent | 17 (17.0) |
| Self-expanding metallic stent | 29 (29.0) |
| Tumour debulking with forceps, laser or argon plasma coagulation | 36 (29.5) |
| Dilatation of stenosis with rigid bronchoscope barrel or balloon dilation | 57 (46.7) |
| Complications of airway intervention | |
| Immediate complications | 25 (20.5) |
| Bleeding >50 mLs | 5 (20.0) |
| Failure to deploy stent | 9 (36.0) |
| Trauma* | 7 (28.0) |
| Others** | 4 (16.0) |
| Complications of airway stenting | 25 (25.0) |
| Stenosis of stent from tumour invasion | 11 (44.0) |
| Stent migration | 9 (36.0) |
| Mucus plugging | 2 (8.0) |
| Granuloma formation | 3 (11.1) |
| Stent infection | 2 (8.0) |
| Repeat airway intervention required | 29 (23.8) |
| Airway stenting | 13 (44.8) |
| Ablation with laser or argon plasma coagulation and/or dilatation | 16 (55.2) |
| Stent removal | 5 (17.2) |
| Repeat tracheostomy | 3 (10.3) |
| Outcomes of airway intervention | |
| Median survival from time of airway intervention in months (IQR) | 3.30 (1.57–6.88) |
| Median survival from time of histological diagnosis in months (IQR) | 8.90 (4.91–14.45) |
| Improvement in respiratory symptoms (n=115) | 96 (83.5) |
| Respiratory-related cause of death (n=97) | 64 (66.0) |
*, one tracheobronchoesophageal fistula from tracheostomy, one pneumothorax post-airway dilatation, one chipped tooth and four with vocal cord edema attributed to rigid bronchoscope intubation; **, myocardial infarction, dislodged esophageal stent, respiratory failure and mucus plugging requiring intubation. IQR, interquartile range.
Straight silicone stents were used in 54/100, 54%, Y silicone stents in 17/100, 17%, and SEMS in 29/100, 29% (Figures 1,3). The proportion of immediate complications with SEMS was 12/29, 41.4%; compared to silicone stents 10/71, 14.1% (P=0.003). Of those who developed delayed post-stenting complications (25/100, 25.0%); 9/25, 36.0% experienced stent migration. Stent migration involved 6 straight silicone stents, 2 covered SEMS and 1 silicone Y stent.
Patients experienced improvement in respiratory symptoms in 96/115, 83.5% (Table 2). The median (IQR) survival from time of histological diagnosis was 8.90 (4.91–14.45) months, while the median (IQR) survival from time of airway intervention was 3.30 (1.57–6.88) months. Death was attributed to respiratory causes in 66%.
Risk factors for tracheobronchoesophageal fistula formation (Table 3, Figure 4)
Table 3
| Variables | No TEF (n=78), n (%) | TEF (n=44), n (%) | P value |
|---|---|---|---|
| Mean age at diagnosis in years ± SD | 61±11 | 62±10 | 0.788 |
| Male gender | 70 (89.7) | 39 (88.6) | 1.000 |
| Stage 3 or 4 at diagnosis | 66 (84.6) | 32 (72.7) | 0.154 |
| Treatment before airway evaluation | |||
| Surgery | 12 (15.4) | 10 (22.7) | 0.311 |
| Chemotherapy | 27 (34.6) | 23 (52.3) | 0.057 |
| Radiotherapy | 17 (21.8) | 25 (56.8) | <0.001 |
| Chemo- and radiotherapy | 13 (16.7) | 18 (40.9) | 0.003 |
| Esophageal stenting | 5 (6.4) | 12 (27.3) | 0.001 |
| Radiotherapy and esophageal stenting | 1 (1.3) | 8 (18.2) | 0.001 |
| Location of airway lesion | |||
| Airway lesion ≤20 mm from carina | 38 (48.7) | 35 (79.5) | 0.001 |
| Location of cancer | |||
| Upper esophageal carcinoma | 23 (29.5) | 10 (22.7) | 0.420 |
| Mid esophageal carcinoma | 46 (58.9) | 32 (72.7) | 0.129 |
| Lower esophageal carcinoma | 9 (11.5) | 2 (4.5) | 0.324 |
| Outcomes after airway intervention | |||
| Respiratory-related cause of death | 34 (57.6)* | 30 (78.9)** | 0.031 |
*, data unavailable for 19 patients; **, data unavailable for 6 patients. TEF, tracheobronchoesophageal fistula; SD, standard deviation.
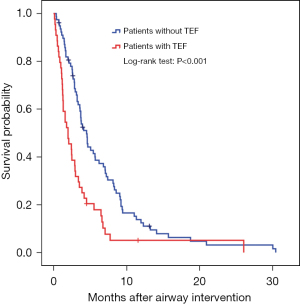
On univariate analysis, radiotherapy and/or prior esophageal stenting were significant risk factors for TEF development (Table 3). Among patients with TEFs, airway lesions ≤20 mm from carina were detected in 35/44, 79.8% as compared to patients without TEF 38/78, 48.7% (P=0.001). Patients with TEF died of respiratory-related causes in 78.9%, as compared to 57.6% of those without TEF (P=0.031). Median (IQR) survival from the time of airway intervention in patients with TEF was 1.98 (0.94–3.02) months compared to patients without TEF, 4.52 (3.56–5.47) months (Figure 4).
Risk factors for survival (Table 4)
Table 4
| Variables | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age at time of intervention | 0.983 (0.966–1.000) | 0.054 | 0.993 (0.970–1.016) | 0.539 | |
| Male gender | 1.422 (0.768–2.633) | 0.263 | 1.588 (0.760–3.317) | 0.219 | |
| Stage 3 or 4 at diagnosis | 1.154 (0.713–1.870) | 0.560 | 1.579 (.837–2.980) | 0.158 | |
| Location of cancer | |||||
| Upper esophageal carcinoma | 1.000 | 1.000 | |||
| Mid esophageal carcinoma | 1.440 (0.940–2.205) | 0.094 | 1.906 (1.133–3.205) | 0.015 | |
| Lower esophageal carcinoma | 0.958 (0.451–2.034) | 0.911 | 0.963 (0.384–2.415) | 0.937 | |
| Airway involvement ≤20 mm from carina | 1.557 (1.060–2.289) | 0.024 | 1.271 (0.789–2.048) | 0.324 | |
| Tracheoesophageal fistula | 2.050 (1.388–3.028) | 0.001 | 1.832 (1.043–3.216) | 0.035 | |
| Surgery before airway intervention | 1.701 (1.062–2.725) | 0.027 | 0.931 (0.499–1.736) | 0.822 | |
| Chemotherapy | |||||
| No chemotherapy | 1.000 | 1.000 | |||
| Chemotherapy before airway intervention | 0.939 (0.618–1.427) | 0.768 | 1.234 (0.712–2.138) | 0.454 | |
| Chemotherapy after airway intervention | 0.384 (0.228–0.649) | 0.001 | 0.459 (0.251–0.838) | 0.011 | |
| Radiotherapy | |||||
| No radiotherapy | 1.000 | 1.000 | |||
| Radiotherapy before airway intervention | 0.937 (0.563–1.560) | 0.803 | 1.053 (0.545–2.032) | 0.878 | |
| Radiotherapy after airway intervention | 0.568 (0.348–0.925) | 0.023 | 0.674 (0.385–1.181) | 0.168 | |
| Esophageal stenting | |||||
| No esophageal stenting | 1.000 | 1.000 | |||
| Esophageal stenting before airway intervention | 1.015 (0.587–1.758) | 0.957 | 0.316 (0.147–0.679) | 0.003 | |
| Esophageal stenting after airway intervention | 0.852 (0.544–1.335) | 0.484 | 0.511 (0.290–0.902) | 0.021 | |
| Airway stenting | |||||
| No airway stenting | 1.000 | 1.000 | |||
| Silicone stent | 1.372 (0.820–2.297) | 0.229 | 1.825 (0.961–3.467) | 0.066 | |
| Self-expanding metallic stent | 1.204 (0.669–2.167) | 0.537 | 1.507 (0.746–3.045) | 0.253 | |
HR, hazard ratio; CI, confidence interval.
On multivariate Cox regression analysis, mid esophageal tumors [adjusted hazard ratio (HR) 1.9; 95% confidence interval (CI): 1.1–3.2] and presence of a TEF (adjusted HR 1.8; 95% CI: 1.0–3.2) were associated with poorer survival (Table 4). Chemotherapy after airway intervention (adjusted HR 0.46; 95% CI: 0.25–0.84), and esophageal stenting either before or after airway intervention (adjusted HR 0.32; 95% CI: 0.15–0.68 and adjusted HR 0.51; 95% CI: 0.29–0.90, respectively) were associated with improved survival.
Discussion
Airway involvement in EC impacts mortality and morbidity (1,2), and there is limited literature from Asia, where the age standardised rate of EC in males is 20.3 per 100,000, almost 30 times that in Europe and North America (21). Our previously published data showed that airway involvement in EC was associated with poorer survival (2). Among EC patients who needed airway intervention, the present study shows that mid EC and TEF were risk factors for poorer survival, while chemotherapy or esophageal stenting were associated with improved survival. Prior radiotherapy and/or esophageal stenting were associated with TEF development. Fistulas were more commonly found within 20 mm of the carina, reflecting anatomical proximity of the carina to the mid esophagus (3,4,22). This explains why our patients with mid EC were more likely to require airway stenting (70/78, 89.7%) and/or esophageal stenting (38/78, 48.7%), as compared to lower and upper EC. The upper esophagus is adjacent to the proximal trachea, which may not always be amenable to airway stenting if disease is in proximity to the glottis. As such, 10/33, 30.3% of our cases with upper EC were managed with a tracheostomy.
The stent migration rate was 9/100 (9%): 8 involving straight silicone stents or SEMS, and 1 involving silicone Y stent. In 3 cases where straight silicone stents were used, the esophageal tumour had regressed following chemotherapy and/or radiotherapy, which led to stent loosening and ultimately dislodgement. As such, it appears prudent to deploy Y-stents for amenable lesions near the carina. Y-stent limbs straddle the carina and anchor the stent. Covered metallic Y-stents are an alternative to silicone stents if expertise and equipment are available (11,23,24).
Esophageal stenting as well as dual stenting of both the esophagus and airway was associated with improved survival in this study. This observation is despite the association between esophageal stents and TEF formation, suggesting that timing of intervention and sequencing with other treatments may be important. Esophageal intervention relieves dysphagia and can improve quality of life over alternatives such as feeding gastrostomy or jejunostomy (1,3,7,25,26). The European Society of Gastrointestinal Endoscopy recommends esophageal SEMS for malignant TEF, and dual stenting if fistula occlusion is not achieved by either esophageal or airway stenting alone (25). Dual stenting in our study was associated with improved survival, consistent with preceding studies (2,6,12). Dual stenting may be advantageous as it affords a better fistula seal and hence fewer aspiration events, as well as reduced risk of airway obstruction from isolated esophageal stenting.
Stent-associated esophago-respiratory fistulas (SERFs) have been shown to occur following 4% of esophageal stenting procedures (27). It is hypothesized that the increased radial force exerted on the esophageal mucosa results in ischemic necrosis. This can be further compounded by radiotherapy. Stent-associated esophago-respiratory fistulas occurred more frequently when proximal or mid EC were stented (27). Similarly, ischemic injury from pressure exerted by both esophageal and airway stents during dual stenting present a theoretical risk. However, when dual esophageal and airway stents were used, a case-control study did not find any relationship with SERF development (odds ratio 1.01, 95% CI: 0.054–18.86) (27), lending support to the use of dual stenting.
Seventeen of 46 (37%) patients had esophageal stenting before airway intervention. Tracheal compression following esophageal stenting has been reported in 7–10% (6,13,28). This can cause respiratory distress necessitating emergent esophageal stent removal, especially in the presence of pre-existing tracheal stenosis or extrinsic compression (13). Two such cases were encountered in this study. In addition, 3 out of 9 patients with failed airway stent deployment had undergone prior esophageal stenting. This highlights that emergent airway stenting in EC has the potential for failure. In another study, 5 out of 7 patients admitted for emergency airway stenting following esophageal stent complications had airway stenting failure (15). Therefore, airway stenting should be performed prior to esophageal stenting during sequential dual stenting. This is especially in patients at risk of airway compromise i.e., airway obstruction known to be >50% of airway lumen.
The risk of TEF formation is higher in patients receiving combined esophageal stenting and radiotherapy, which is the reason guidelines do not recommend radiotherapy after esophageal stenting (25,27,29-31). Additionally, randomized controlled trial data show limited improvement in dysphagia or survival with the addition of radiotherapy following esophageal stenting (32). However, data demonstrate the safety of catheter-based brachytherapy when administered after insertion of esophageal SEMS (33,34). This may be considered as an alternative treatment modality.
The present study reflects the largest number of patients with EC requiring airway intervention to date. Limitations include the retrospective nature of the study, which spanned 20 years as treatment of advanced EC continued to evolve. However, the single centre experience and airway interventions performed by a small group of interventional pulmonologists have ensured completeness of data collection and relative standardization of endoscopic technique.
To date, there is still insufficient data to guide an evidence based approach to airway intervention in advanced EC. To bridge this gap, we summarized the findings from this study, as well as a review of literature into a management algorithm as shown in Figures 5,6. First, there should be a low clinical threshold to suspect airway involvement in patients with EC, and careful bronchoscopic evaluation needs to be undertaken in these patients. This will facilitate early detection of extrinsic compression and mucosal invasion, which should be distinguished from each other. If in doubt, radial endobronchial ultrasonography may be useful to assess extent of airway wall invasion (35,36). Mucosal involvement often results in mixed i.e., extrinsic and intrinsic obstruction, which requires multi-modal therapy. We propose close airway surveillance of non-critical (≤50%) extrinsic compression (37), and surgical options may be explored in appropriate candidates in the absence of mural invasion. In cases with significant (>50%) airway stenosis (37), airway and esophageal dual stenting should be considered. In our practice, airway stents should extend at least 5mm beyond the proximal and distal end of the lesion to ensure adequate coverage. The best fitting stents should be used, as oversized stents risk further fistula extension, stent erosion and compression of adjacent structures. Y stenting is preferred for lesions within 20 mm of the carina to minimise stent migration. Esophageal stenting may proceed if the tumour is deemed unlikely to further compromise airway patency after insertion of the esophageal stent.
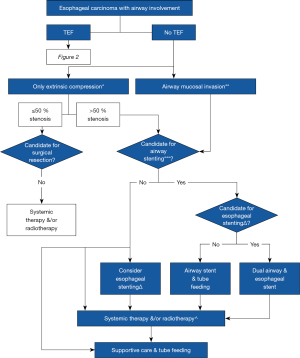
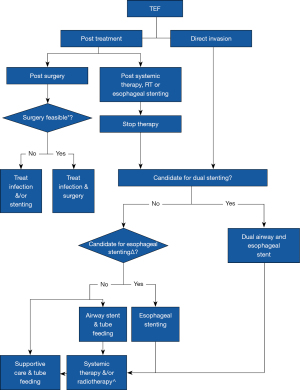
Elective dual airway and esophageal stenting in cases with TEF, and significant tracheobronchial stenosis or mucosal invasion should be considered. Covered SEMS can provide a good TEF seal due to their expandable nature. Airway stents should be placed first during dual stenting to avoid potential airway compromise. Adjunctive systemic therapy including immuno- or chemotherapy may control disease progression post airway intervention (1), and close tracheobronchial surveillance is advisable during the course of oncological therapy. Radiotherapy to the esophagus after stenting should be used with caution. Brachytherapy may be an alternative or used in addition to esophageal stenting for malignant dysphagia (1). Palliation with supportive care, including tube feeding, should also be considered in patients deemed unsuitable for more invasive therapies.
Conclusions
This study represents the largest published cohort of patients with EC and airway involvement to date. The middle esophagus is in close proximity to the airway, and tumours at this location can carry greater morbidity and requirement for airway interventions. Factors associated with TEF formation include location of the tumour in the airways, and prior radiotherapy and/or esophageal stenting. Fistula development was associated with poorer survival. Therefore, careful bronchoscopic evaluation and surveillance is necessary in patients with EC and suspected airway involvement. Y silicone rather than straight stents are preferred for lesions within 20 mm of the carina. Dual stenting affords better fistula seal and should be considered for the management TEF. These findings have been summarised into a proposed treatment algorithm that should be revised as new data emerges.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-138/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-138/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-138/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of SingHealth CIRB C (approval number: 2017-2966). Requirement for informed consent was waived, as the study was retrospective in nature and data was de-identified prior to analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McMillian N, Lenora Pluchino MA, Ajani JA, et al. NCCN Guidelines Version 5.2020 Esophageal and Esophagogastric Junction Cancers Continue NCCN. 2020.
- Goh KJ, Lee P, Foo AZX, et al. Characteristics and Outcomes of Airway Involvement in Esophageal Cancer. Ann Thorac Surg 2021;112:912-20. [Crossref] [PubMed]
- Balazs A, Galambos Z, Kupcsulik PK. Characteristics of esophagorespiratory fistulas resulting from esophageal cancers: a single-center study on 243 cases in a 20-year period. World J Surg 2009;33:994-1001. [Crossref] [PubMed]
- Duranceau A, Jamieson GG. Malignant tracheoesophageal fistula. Ann Thorac Surg 1984;37:346-54. [Crossref] [PubMed]
- Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am 2003;13:271-89. [Crossref] [PubMed]
- Herth FJ, Peter S, Baty F, et al. Combined airway and oesophageal stenting in malignant airway-oesophageal fistulas: a prospective study. Eur Respir J 2010;36:1370-4. [Crossref] [PubMed]
- Shin JH, Song HY, Ko GY, et al. Esophagorespiratory fistula: long-term results of palliative treatment with covered expandable metallic stents in 61 patients. Radiology 2004;232:252-9. [Crossref] [PubMed]
- Murthy S, Gonzalez-Stawinski GV, Rozas MS, et al. Palliation of malignant aerodigestive fistulae with self-expanding metallic stents. Dis Esophagus 2007;20:386-9. [Crossref] [PubMed]
- Rodriguez AN, Diaz-Jimenez JP. Malignant respiratory-digestive fistulas. Curr Opin Pulm Med 2010;16:329-33. [Crossref] [PubMed]
- Dumonceau JM, Cremer M, Lalmand B, et al. Esophageal fistula sealing: choice of stent, practical management, and cost. Gastrointest Endosc 1999;49:70-8. [Crossref] [PubMed]
- Li TF, Duan XH, Han XW, et al. Application of combined-type Y-shaped covered metallic stents for the treatment of gastrotracheal fistulas and gastrobronchial fistulas. J Thorac Cardiovasc Surg 2016;152:557-63. [Crossref] [PubMed]
- Freitag L, Tekolf E, Steveling H, et al. Management of malignant esophagotracheal fistulas with airway stenting and double stenting. Chest 1996;110:1155-60. [Crossref] [PubMed]
- Nomori H, Horio H, Imazu Y, et al. Double stenting for esophageal and tracheobronchial stenoses. Ann Thorac Surg 2000;70:1803-7. [Crossref] [PubMed]
- Colt HG, Meric B, Dumon JF. Double stents for carcinoma of the esophagus invading the tracheo-bronchial tree. Gastrointest Endosc 1992;38:485-9. [Crossref] [PubMed]
- Paganin F, Schouler L, Cuissard L, et al. Airway and esophageal stenting in patients with advanced esophageal cancer and pulmonary involvement. PLoS One 2008;3:e3101. [Crossref] [PubMed]
- Shin B, Chang B, Kim H, et al. Interventional bronchoscopy in malignant central airway obstruction by extra-pulmonary malignancy. BMC Pulm Med 2018;18:46. [Crossref] [PubMed]
- Freitas C, Serino M, Cardoso C, et al. Predictors of survival and technical success of bronchoscopic interventions in malignant airway obstruction. J Thorac Dis 2021;13:6760-8. [Crossref] [PubMed]
- Hsu AAL, Lee P. Interventional Bronchoscopy and Pleuroscopy. Singapore: World Scientific; 2018.
- Standring S. Gray’s Anatomy. 42nd Edition. Philadelphia: Elsevier; 2020
- AJCC - Cancer Staging Manual, Available online: https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspx (accessed 6 June 2021).
- Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer 2012;31:281-6. [Crossref] [PubMed]
- Martini N, Goodner JT, D'Angio GJ, et al. Tracheoesophageal fistula due to cancer. J Thorac Cardiovasc Surg 1970;59:319-24. [Crossref] [PubMed]
- Gompelmann D, Eberhardt R, Schuhmann M, et al. Self-expanding Y stents in the treatment of central airway stenosis: a retrospective analysis. Ther Adv Respir Dis 2013;7:255-63. [Crossref] [PubMed]
- Madan K, Dhooria S, Sehgal IS, et al. A Multicenter Experience With the Placement of Self-Expanding Metallic Tracheobronchial Y Stents. J Bronchology Interv Pulmonol 2016;23:29-38. [Crossref] [PubMed]
- Spaander MC, Baron TH, Siersema PD, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016;48:939-48. [Crossref] [PubMed]
- Chen YH, Li SH, Chiu YC, et al. Comparative study of esophageal stent and feeding gastrostomy/jejunostomy for tracheoesophageal fistula caused by esophageal squamous cell carcinoma. PLoS One 2012;7:e42766. [Crossref] [PubMed]
- Bick BL, Song LM, Buttar NS, et al. Stent-associated esophagorespiratory fistulas: incidence and risk factors. Gastrointest Endosc 2013;77:181-9. [Crossref] [PubMed]
- Kim KR, Shin JH, Song HY, et al. Palliative treatment of malignant esophagopulmonary fistulas with covered expandable metallic stents. AJR Am J Roentgenol 2009;193:W278-82. [Crossref] [PubMed]
- Nishimura Y, Nagata K, Katano S, et al. Severe complications in advanced esophageal cancer treated with radiotherapy after intubation of esophageal stents: a questionnaire survey of the Japanese Society for Esophageal Diseases. Int J Radiat Oncol Biol Phys 2003;56:1327-32. [Crossref] [PubMed]
- Zhong J, Wu Y, Xu Z, et al. Treatment of medium and late stage esophageal carcinoma with combined endoscopic metal stenting and radiotherapy. Chin Med J (Engl) 2003;116:24-8. [PubMed]
- Javed A, Pal S, Dash NR, et al. Palliative stenting with or without radiotherapy for inoperable esophageal carcinoma: a randomized trial. J Gastrointest Cancer 2012;43:63-9. [Crossref] [PubMed]
- Adamson D, Byrne A, Porter C, et al. Palliative radiotherapy after oesophageal cancer stenting (ROCS): a multicentre, open-label, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol 2021;6:292-303. [Crossref] [PubMed]
- Bergquist H, Johnsson E, Nyman J, et al. Combined stent insertion and single high-dose brachytherapy in patients with advanced esophageal cancer - results of a prospective safety study. Dis Esophagus 2012;25:410-15. [Crossref] [PubMed]
- Amdal CD, Jacobsen AB, Sandstad B, et al. Palliative brachytherapy with or without primary stent placement in patients with oesophageal cancer, a randomised phase III trial. Radiother Oncol 2013;107:428-33. [Crossref] [PubMed]
- Herth F, Ernst A, Schulz M, et al. Endobronchial ultrasound reliably differentiates between airway infiltration and compression by tumor. Chest 2003;123:458-62. [Crossref] [PubMed]
- Wakamatsu T, Tsushima K, Yasuo M, et al. Usefulness of preoperative endobronchial ultrasound for airway invasion around the trachea: esophageal cancer and thyroid cancer. Respiration 2006;73:651-7. [Crossref] [PubMed]
- Freitag L, Ernst A, Unger M, et al. A proposed classification system of central airway stenosis. Eur Respir J 2007;30:7-12. [Crossref] [PubMed]


