
Comparison of efficacy and safety of hybrid video-assisted thoracoscopic surgery vs. thoracotomy sleeve lobectomy for non-small cell lung cancer: a propensity score matching study
Introduction
Lung cancer is one of the leading causes of cancer-related morbidity and mortality in the world (1). Sleeve lobectomy (SL), which is an alternative approach to pneumonectomy, is popular among both thoracic surgeons and some patients with centrally-located lung cancer due to its ability to achieve the same oncological result with more lung parenchyma preservation. Recent articles have demonstrated that SL is a more effective surgical approach than pneumonectomy for treatment of anatomically suitable lung cancer patients, with better short- and long-term survival outcomes (2,3). Improvements in pulmonary function and quality of life have also been reported in selected patients who underwent SL instead of pneumonectomy (4,5). However, bronchial and/or vascular anastomoses in SL procedures are technically challenging, especially under complete video-assisted thoracoscopic surgery (VATS).
Hybrid VATS is a minimally invasive approach which lies between standard thoracotomy and video-assisted surgery (6). The advantages of hybrid VATS are direct visualization of the surgical field and flexible maneuverability (6). A few articles have reported that hybrid VATS SL is a secure, minimally invasive, and technically feasible approach for selected patients (6,7). However, none of them evaluated the safety and efficacy compared with thoracotomy SL.
In this retrospective study, we collected, analyzed, and compared the clinical data of patients who underwent SL under either standard thoracotomy or hybrid VATS at the Cancer Hospital of Dalian University of Technology, Liaoning Cancer Hospital & Institute, Shenyang. We focused on mortality, overall survival (OS), recurrence-free survival (RFS), and postoperative complications between the two groups. We hypothesized that hybrid SL could achieve same oncologic result as standard thoracotomy and was a feasible and safe procedure. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-754/rc).
Methods
Study population
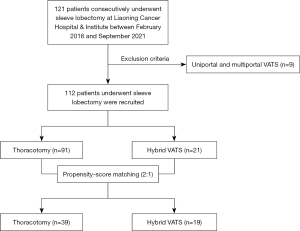
A total of 121 consecutive patients who underwent sleeve resection for primary treatment of NSCLC from November 2016 to September 2021 at Liaoning Cancer Hospital & Institute were included in our study. We excluded 9 patients who underwent single or multiple ports VATS SL. Finally, 112 patients were recruited and divided into two groups according to surgical approaches (Figure 1). The clinical data were collected and retrospectively analyzed. Preoperative examinations were routinely conducted including enhanced high-resolution computed tomography (HRCT), bronchoscopy, abdominal ultrasound, enhanced brain magnetic resonance imaging (MRI), and bone scintigraphy. Positron emission tomography (PET) scan, endobronchial ultrasound (EBUS)-guided biopsy, and mediastinoscopy were mostly used when suspected positive mediastinal lymph nodes were detected by computed tomography (CT) scan. All patients were staged according to the eighth edition of tumor-node-metastasis (TNM) classification for lung cancer (8). Neoadjuvant therapy was recommended for patients with resectable c-stage II and III non-small cell lung cancer (NSCLC). Adjuvant therapy was routinely given to patients who were p-stage II or III. Complications were evaluated using the Clavien-Dindo grading system. The follow-up data were obtained by outpatient clinical visit and nurse-led telephone follow-up. We defined OS as the time interval between the date of surgery and that of death. We defined RFS as the time interval between the date of surgery and that of recurrence or last follow-up.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Liaoning Cancer Hospital & Institute Ethics Committee (No. 202205128). Written informed consent was waived by the ethics committee due to retrospective nature of the study.
Surgical procedure
The surgical incision length ranged from 5 to 10 cm, and typically about 8 cm. The incision was located in the fourth or fifth intercostal space according to the location of tumor (Figure 2). An additional 1 cm long incision was made in the eighth intercostal space as a camera port which was used for drainage after surgery. An incision protection retractor was routinely used without rib spreading. Head-mounted light was usually used for narrow surgical view enhancement during the hybrid VATS SL procedure. A rib retractor was only used to achieve a smooth anastomose of vessels when the surgical view remained too narrow even with the aid of a head-mounted light. The detailed procedure of hybrid VATS was conducted as previously described (9).
The definition of thoracotomy is a conventional posterolateral thoracotomy with rib spreading and large incision about 15–20 cm in length at the fourth or fifth intercostal space.
Statistical analysis
Categorical variables were expressed as number (proportion) and were analyzed by Pearson’s chi-square test and Fisher’s exact test. Continuous variables were expressed as mean ± standard deviation (SD) and analyzed by Student’s t-test. Propensity score matching (PSM) was used to control the baseline characteristics and achieve a better homogeneity of the two groups. The OS and RFS were analyzed using Kaplan-Meier method and log-rank test.
Statistical analysis was performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA) and R studio software (version 4.1.0; The R Foundation for Statistical Computing, Vienna, Austria). The level of statistical significance was set at a two-sided P value <0.05.
Results
The baseline characteristics of all cases are summarized in Table 1. The baseline data of patients was quite similar between the two groups, except regarding the receipt of neoadjuvant therapy. No differences were observed in age, gender, smoking history, comorbidities, tumor location, tumor size, histology, double SL (DSL), extended SL (ESL) or SL, R0 resection, and adjuvant therapy between the two groups. Additionally, the proportion of patients who received neoadjuvant therapy in the hybrid VATS group was higher than that among patients in the thoracotomy group, but no significantly difference was found (P=0.087).
Table 1
| Characteristics | Thoracotomy (n=91) | Hybrid VATS (n=21) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 60.1±8.3 | 59.7±9.0 | 0.859 |
| Gender | 0.753 | ||
| Male | 76 | 17 | |
| Female | 15 | 4 | |
| Smoking history | 0.780 | ||
| Yes | 69 | 15 | |
| No | 22 | 6 | |
| Comorbidities | 0.585 | ||
| Yes | 23 | 7 | |
| No | 68 | 14 | |
| Tumor location | 0.380 | ||
| RUL | 64 | 16 | |
| RML + RLL | 3 | 2 | |
| LUL | 1 | 0 | |
| LLL | 23 | 3 | |
| Tumor size (cm), mean ± SD | 3.4±2.1 | 3.1±1.3 | 0.474 |
| Histology | 0.489 | ||
| Squamous cell carcinoma | 68 | 17 | |
| Adenocarcinoma | 11 | 3 | |
| Others | 12 | 1 | |
| pTNM stage | 0.587 | ||
| I | 21 | 4 | |
| II | 43 | 8 | |
| III | 26 | 9 | |
| IV | 1 | 0 | |
| Neoadjuvant therapy | 0.087 | ||
| Yes | 31 | 12 | |
| No | 60 | 9 | |
| R status | 0.500 | ||
| R0 | 79 | 16 | |
| Run | 10 | 4 | |
| R1 | 2 | 1 | |
| Adjuvant therapy | 0.732 | ||
| Yes | 77 | 19 | |
| No | 14 | 2 | |
| DSL | 0.219 | ||
| Yes | 8 | 3 | |
| No | 83 | 18 | |
| ESL or SL | 0.743 | ||
| SL | 77 | 17 | |
| ESL | 14 | 4 |
VATS, video-assisted thoracoscopic surgery; SD, standard deviation; RUL, right upper lung; RML, right mid-lung; RLL, right lower lung; LUL, left upper lung; LLL, left lower lung; pTNM, pathological tumor-node-metastasis; DSL, double sleeve lobectomy; ESL, extended sleeve lobectomy; SL, sleeve lobectomy.
The perioperative data are shown in Table 2. Patients in the hybrid VATS group had longer operation time compared with patients in the thoracotomy group (P<0.01). The number of harvested lymph nodes and positive lymph nodes were significantly higher in the hybrid VATS group than the thoracotomy group (P<0.05). Significantly shorter postoperative hospital stay and chest tube duration time were observed in the hybrid VATS group compared with the thoracotomy group (P<0.05). The number of dissected lymph node stations was higher in the hybrid group, but not significantly different between the two groups (P=0.061). Complications were not significantly different between the two groups (P=0.657). The reoperation and recurrence rates were similar between hybrid SL and thoracotomy SL groups (P=1.0, P=0.519). The 30- and 90-day mortalities were similar between two groups (1.1% vs. 0%, P=1.0; 3.3% vs. 4.8%, P=0.57).
Table 2
| Variables | Thoracotomy (n=91) | Hybrid VATS (n=21) | P value |
|---|---|---|---|
| Operation time (min), mean ± SD | 271.5±93.5 | 332.4±60.5 | <0.01 |
| Number of lymph node stations, mean ± SD | 9.2±1.5 | 9.9±1.4 | 0.061 |
| Number of lymph nodes, mean ± SD | 24.8±7.8 | 32.3±9.4 | <0.01 |
| Number of positive lymph nodes, mean ± SD | 3.2±3.2 | 5.0±4.2 | 0.04 |
| Complications (Clavien-Dindo classification) | 0.657 | ||
| Grade 0–I | 41 | 9 | |
| Grade II | 36 | 7 | |
| Grade III | 12 | 4 | |
| Grade IV–V | 2 | 1 | |
| Postoperative stay (days), mean ± SD | 10.6±4.4 | 6.9±2.5 | 0.003 |
| Chest tube duration time (days), mean ± SD | 7.0±3.2 | 4.9±1.5 | 0.016 |
| Mortality within 30 days | 1.1% | 0% | 1.0 |
| Yes | 1 | 0 | |
| No | 90 | 21 | |
| Mortality within 90 days | 3.3% | 4.8% | 0.57 |
| Yes | 3 | 1 | |
| No | 88 | 20 | |
| Reoperation | 4.4% | 4.8% | 1.0 |
| Yes | 4 | 1 | |
| No | 87 | 20 | |
| Recurrence | 18.7% | 9.5% | 0.519 |
| Yes | 17 | 2 | |
| No | 74 | 19 |
VATS, video-assisted thoracoscopic surgery; SD, standard deviation.
After PSM, 39 patients in the thoracotomy group and 19 patients in the hybrid VATS group were matched. The baseline characteristics of patients are listed in Table 3. The baseline variables were quite similar in both groups. The perioperative variables are shown in Table 4. In the hybrid group, the number of lymph nodes and positive lymph nodes were significantly higher in comparison with those of the thoracotomy group (P<0.05). The number of dissected lymph nodes stations was slightly higher in the hybrid group compared with that of thoracotomy group, but not significantly higher (P=0.262). Significantly shorter postoperative hospital stay and chest tube duration time were found in the hybrid group compared with the thoracotomy group (P<0.05). The operation time was similar between two groups (295.2±114.7 vs. 331.8±63.4 minutes, P=0.123). No statistical differences in complications, reoperation, and recurrence were observed between the hybrid VATS SL and thoracotomy SL (P=1.0, P=1.0, P=0.472). The 30- and 90-day mortalities were not significantly different between the two groups (2.6% vs. 0%, P=1.0; 5.1% vs. 5.3%, P=1.0).
Table 3
| Characteristics | Thoracotomy (n=39) | Hybrid VATS (n=19) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 60.0±9.8 | 60.0±9.2 | 0.916 |
| Gender | 1.0 | ||
| Male | 30 | 15 | |
| Female | 9 | 4 | |
| Smoking history | 1.0 | ||
| Yes | 28 | 13 | |
| No | 11 | 6 | |
| Comorbidities | 1.0 | ||
| Yes | 11 | 6 | |
| No | 28 | 13 | |
| Tumor location | 0.416 | ||
| RUL | 28 | 15 | |
| RML + RLL | 1 | 2 | |
| LUL | 1 | 0 | |
| LLL | 9 | 2 | |
| Tumor size (cm), mean ± SD | 3.6±2.3 | 3.0±1.3 | 0.331 |
| Histology | 1.0 | ||
| Squamous cell carcinoma | 30 | 15 | |
| Adenocarcinoma | 6 | 3 | |
| Others | 3 | 1 | |
| pTNM stage | 0.835 | ||
| I | 6 | 4 | |
| II | 19 | 8 | |
| III | 14 | 7 | |
| Neoadjuvant therapy | 0.745 | ||
| Yes | 25 | 13 | |
| No | 14 | 6 | |
| R status | 1.0 | ||
| R0 | 31 | 16 | |
| Run | 6 | 2 | |
| R1 | 2 | 1 | |
| Adjuvant therapy | 0.591 | ||
| Yes | 37 | 17 | |
| No | 2 | 2 | |
| DSL | 0.704 | ||
| Yes | 5 | 3 | |
| No | 35 | 16 | |
| ESL or SL | 1.0 | ||
| SL | 34 | 16 | |
| ESL | 5 | 3 |
PSM, propensity-score matching; VATS, video-assisted thoracoscopic surgery; SD, standard deviation; RUL, right upper lung; RML, right mid-lung; RLL, right lower lung; LUL, left upper lung; LLL, left lower lung; pTNM, pathological tumor-node-metastasis; DSL, double sleeve lobectomy; ESL, extended sleeve lobectomy; SL, sleeve lobectomy.
Table 4
| Variables | Thoracotomy (n=39) | Hybrid VATS (n=19) | P value |
|---|---|---|---|
| Operation time (min), mean ± SD | 295.2±114.7 | 331.8±63.4 | 0.123 |
| Number of lymph node stations, mean ± SD | 9.3±1.5 | 9.8±1.4 | 0.262 |
| Number of lymph nodes, mean ± SD | 25.9±8.5 | 32.9±9.7 | <0.01 |
| Number of positive lymph nodes, mean ± SD | 3.7±2.9 | 5.6±4.0 | 0.045 |
| Complications (Clavien-Dindo classification) | 1.0 | ||
| Grade 0–I | 15 | 8 | |
| Grade II | 16 | 7 | |
| Grade III | 6 | 3 | |
| Grade IV–V | 1 | 1 | |
| Postoperative stay (days), mean ± SD | 9.5±3.5 | 7.3±2.9 | 0.021 |
| Chest tube duration time (days), mean ± SD | 6.6±3.1 | 5.3±1.5 | 0.031 |
| Mortality within 30 days | 2.6% | 0% | 1.0 |
| Yes | 1 | 0 | |
| No | 38 | 19 | |
| Mortality within 90 days | 5.1% | 5.3% | 1.0 |
| Yes | 2 | 1 | |
| No | 37 | 18 | |
| Reoperation | 5.1% | 0% | 1.0 |
| Yes | 2 | 0 | |
| No | 37 | 19 | |
| Recurrence | 20.5% | 10.5% | 0.472 |
| Yes | 8 | 2 | |
| No | 31 | 17 |
PSM, propensity-score matching; VATS, video-assisted thoracoscopic surgery; SD, standard deviation.
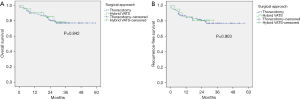
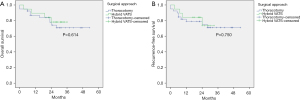
The median follow-up time of the whole cohort was 30 months. A total of 23 patients died and 19 patients experienced recurrence during the follow-up period. There were no significant differences between open and hybrid VATS SL in 3-year OS (P=0.842) and 3-year RFS (P=0.903) before PSM (Figure 3). No significant differences were observed between the two groups in 3-year OS (P=0.614) and 3-year RFS (P=0.750) after PSM (Figure 4).
Discussion
The first attempt of SL for lung cancer was made by Allison in 1954 (10). Initially, SL was considered a compromise surgical approach for patients who could not tolerate pneumonectomy. Currently, SL is considered an alternative approach to pneumonectomy to achieve the same oncological results, better survival results, and improved quality of life. Gao et al. showed that VATS SL is a safe and effective procedure with satisfactory postoperative and oncologic results for anatomically suitable lung cancer patients (11). Xie et al. demonstrated that uniportal SL with pulmonary arterioplasty was a safe and feasible procedure after careful selection of centrally located patients by experienced surgeons (12). Previously, DSL was seen as a very complex surgical procedure for most thoracic surgeons. Wu et al. demonstrated that uniportal DSL was feasible and effective, reduced the postoperative pain, and achieved similar postoperative results (13). However, in their study, only 42 patients received DSL, and only 21 patients were included in the uniportal group. It was proposed that uniportal SL could only be performed by experienced surgeons in large centers, making it difficult to widely applied even in most of large centers.
Compared to uniportal SL, hybrid VATS SL was relatively easier and could be widely applied with intensive training and accumulation of surgical experience. In addition, the hybrid VATS approach could be safely used for pulmonary resection, even at community hospitals (14). He et al. demonstrated that hybrid VATS SL was feasible and safe for selected patients with bronchogenic tumors through a single center study (7).
Our hybrid SL approach was different from that used in other studies; a rib retractor was used without rib spreading in our hybrid VATS approach, the damage from which is theoretically smaller than that of other hybrid VATS approaches. About an 8 cm hybrid VATS incision without rib spreading is usually enough for most sleeve resections including DSL at our hospital. Only a small number of studies (6,7) had demonstrated the potential benefits of hybrid VATS SL, and one study (6) was in comparison with thoracotomy SL, but there had been potential bias for the baseline characteristics among the study groups. Therefore, this retrospective study was designed to directly compare both approaches.
The operation time was similar between the two groups, indicating that hybrid VATS SL did not require additional surgical time. More lymph nodes were harvested and more positive lymph nodes were diagnosed via hybrid VATS. Therefore, it could help to more accurately diagnose of the lymph nodes, and more accurate treatment was achieved herein. Hybrid VATS SL was associated with better perioperative results in our study. Chest tube duration and postoperative hospital stay time was shorter in the hybrid VATS group, indicating that the postoperative recovery was faster in the hybrid VATS SL group. Therefore, hybrid VATS SL enhanced postoperative recovery with smaller incisions and less associated trauma in comparison with standard thoracotomy. Similar OS and RFS were observed before and after PSM between the two groups. Hybrid VATS SL did not compromise oncological results compared with thoracotomy SL. In summary, the results of our study highlight the merits and meaningfulness of the hybrid SL approach.
Hybrid VATS SL is more straightforward for most surgeons because it can provide an excellent operative field. It can be widely applied with intensive training and accumulation of surgical experience. It is relatively easier for surgeons to perform SL under hybrid VATS with accumulation of surgical experience, and even the more complex procedure, SL with vascular reconstruction, than under VATS or robotic-assisted thoracoscopic surgery (RATS). In our study, SL was performed by two experienced surgeons who had performed more than 1,000 cases of VATS lobectomies and 60 cases of thoracotomy SL at our hospital. In our experience, at least 100 cases of VATS lobectomies and 10 cases of thoracotomy sleeve resection should be accumulated to lay the anatomical and technical foundation before performing hybrid VATS SL.
There were several limitations to our study. First, the nature of the study was retrospective, thus selection bias was inevitable. Second, although we used PSM to minimize the potential selection bias, there were also other confounders which might have had an impact on the final results, such as patient will and economic reasons, which could still affect the patient’s selection. Third, this was a single-center based study, and the number of patients was small, especially that of patients in the hybrid group. Therefore, a multicenter, large sample size, randomized controlled trial is needed to further validate the conclusions from our study in the future.
Conclusions
Hybrid VATS SL may be a feasible, safe, and effective approach for NSCLC. Hybrid VATS SL is associated with a higher number of lymph nodes harvested, more positive lymph nodes detected, better postoperative recovery, and the same oncological results as those of thoracotomy SL.
Acknowledgments
Funding: This study was supported by grants from the Natural Science Foundation Guidance Program of Liaoning Province (No. 2019-ZD-0589); Department of Liaoning Science and Technology, titled with the Construction of Liaoning Cancer Research Center (Lung Cancer) (No. 1564992449013); Precise Diagnosis and Treatment and Optimization of a Clinical Pathway for Malignant Tumor Based on Molecular Markers the Research of Precise Treatment and Optimization of Clinical Pathways for Lung Cancer (No. 2019020176-JH1/103-02); Technological Special Project of Liaoning Province of China (No. 2019020176-JH1/103); Central Financial Fund for Promoting Medical Service and Safeguarding Capability (Capability Construction of Medical and Health Organizations)—a subsidy to the Construction of Provincial Key Specialty; Research Grant to Introduced Talents of Liaoning Cancer Hospital.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-754/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-754/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-754/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Liaoning Cancer Hospital & Institute Ethics Committee (No. 202205128). Written informed consent was waived by the ethics committee due to retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref] [PubMed]
- Chen J, Soultanis KM, Sun F, et al. Outcomes of sleeve lobectomy versus pneumonectomy: A propensity score-matched study. J Thorac Cardiovasc Surg 2021;162:1619-28.e4. [Crossref] [PubMed]
- Li Z, Chen W, Xia M, et al. Sleeve lobectomy compared with pneumonectomy for operable centrally located non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2019;8:775-86. [Crossref] [PubMed]
- Park JS, Yang HC, Kim HK, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol 2010;5:517-20. [Crossref] [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life after lung cancer surgery: a prospective pilot study comparing bronchial sleeve lobectomy with pneumonectomy. J Thorac Oncol 2008;3:604-8. [Crossref] [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Hybrid surgical approach of video-assisted minithoracotomy for lung cancer: significance of direct visualization on quality of surgery. Chest 2005;128:2696-701. [Crossref] [PubMed]
- He J, Shao W, Cao C, et al. Long-term outcome of hybrid surgical approach of video-assisted minithoracotomy sleeve lobectomy for non-small-cell lung cancer. Surg Endosc 2011;25:2509-15. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Zhang C, Yu Z, Li J, et al. Hybrid video-assisted thoracoscopic surgery sleeve lobectomy for non-small cell lung cancer: a case report. J Thorac Dis 2020;12:6836-46. [Crossref] [PubMed]
- Allison PR. Course of thoracic surgery in Groningen. Ann R Coll Surg 1954;25:20-2.
- Gao HJ, Jiang ZH, Gong L, et al. Video-Assisted Vs Thoracotomy Sleeve Lobectomy for Lung Cancer: A Propensity Matched Analysis. Ann Thorac Surg 2019;108:1072-9. [Crossref] [PubMed]
- Xie D, Zhong Y, Deng J, et al. Comparison of uniportal video-assisted thoracoscopic versus thoracotomy bronchial sleeve lobectomy with pulmonary arterioplasty for centrally located non-small-cell lung cancer. Eur J Cardiothorac Surg 2021;59:978-86. [Crossref] [PubMed]
- Wu L, Wang H, Cai H, et al. Comparison of Double Sleeve Lobectomy by Uniportal Video-Assisted Thoracic Surgery (VATS) and Thoracotomy for NSCLC Treatment. Cancer Manag Res 2019;11:10167-74. [Crossref] [PubMed]
- Kim RH, Takabe K, Lockhart CG. A hybrid technique: video-assisted thoracoscopic surgery (VATS) pulmonary resections for community-based surgeons. Surg Endosc 2010;24:700-4. [Crossref] [PubMed]
(English Language Editor: J. Jones)


