
Clinicopathological analysis of 241 thymic epithelial tumors—experience in the Shanghai Chest Hospital from 1997–2004
Introduction
Thymic epithelial tumors (TET) comprise of thymomas and thymic carcinomas (TCs). Due to their rarity, heterogeneous morphology, and equivocal diagnostic criteria, histological classification of TET has been controversial (1,2). Since 1999 the WHO classification labels the main thymoma histological subtype as A, AB, B1, B2 and B3 (3,4). Although well-established world-wide, its prognostic value has been under debate (5-8). Most studies (9), including one of 200 cases treated between 1969 and 1996 at Shanghai Chest Hospital (SCH) (8), found A, AB and B1 thymomas to have a better prognosis, while others reported on comparable aggressiveness of B1, B2 and B3 thymomas (7), and aggressive and unresectable cases of A thymomas (6). Hereby, we investigated 244 new patients with thymomas and TCs from the SCH that were treated more than a decade later and compared clinicopathological variables and outcome in the two cohorts.
Materials and methods
Sources of data
A total of 335 consecutive patients underwent surgical resection of TET at SCH from 1997 to 2004. Among them, 244 patients with available treatment and follow-up data were included into the study. All histological sections were reviewed and tumors were classified according to the 2004 WHO classification of thymic tumor (4). All histological sections were first reviewed by 2 senior pathologists (Jie Zhang, Lei Zhu) separately according to the 2004 WHO classification of thymic tumor (4). Tumor sections from all patients who were dead, and some typical and debatable cases (n=110, 45.6% of the cohort) were reviewed by Alexander Marx. Consensus was achieved at a face-to-face microscopy session (J.Z., L.Z., A.M.).
There were 183 thymomas, 58 TCs. Two meta-plastic and 1 micronodular thymoma were excluded for further analysis. The study was approved by the Institutional Review Board of SCH. Clinicopathological data was retrieved from of SCH’s files. The deadline of follow-up was June 30th, 2011. The time of follow-up ranged from 6.4 to 14.5 years (median: 7.8 years).
Statistical analysis
Data of 241 patients was statistically analyzed by SPSS 18 statistic software (SPSS Inc., Chicago) and SAS software, release 9.2 (SAS Institute Inc., Cary, NC, USA). Qualitative parameters were presented by their absolute and relative frequencies; for quantitative variables mean values ± standard deviation together with the corresponding ranges were given. In order to compare several groups regarding a qualitative parameter, Chi-square test or Fisher’s exact test was used, as appropriate. Mean values were compared by 1-one-ANOVAs; for comparisons of two groups, 2-sample t-tests was used.
Overall survival (OS) and progression-free survival (PFS) were analyzed by the Kaplan-Meier method and evaluated for statistical differences by the log-rank test. Multivariate Cox regression analysis was used to investigate simultaneously the effects of possible risk factors [gender, age, myasthenia gravis (MG) status, tumor size, histotype, Masaoka stage, resection status, and (neo-adjuvant) treatment] on survival. Results were considered as statistically significant for P<0.05. For multiple Cox regression models significance level was set at 0.10.
Results
Clinicopathologic characteristics of current cases
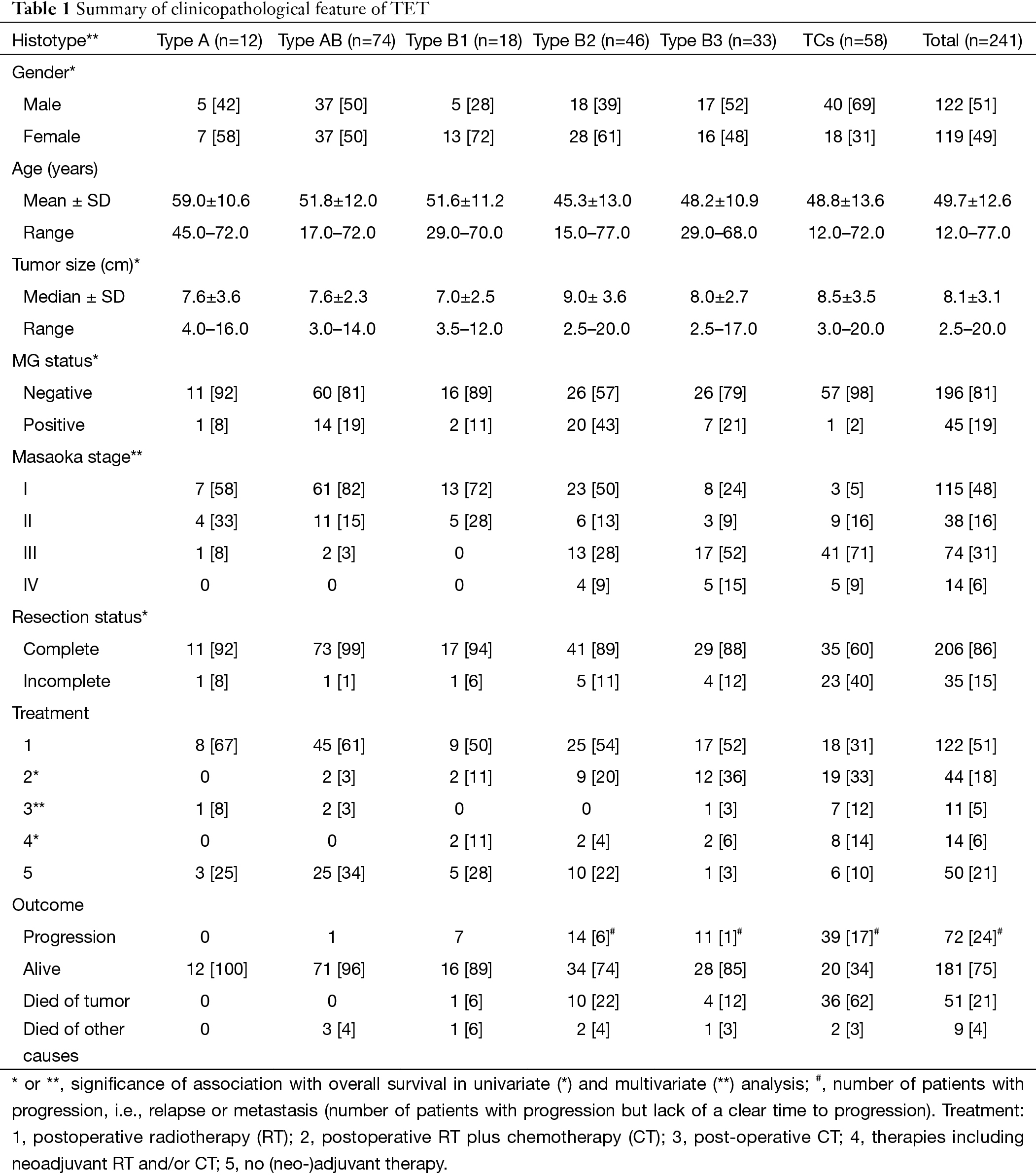
Details of the tumors and patients are given in Table 1. In TCs, the proportion of male patients was higher than that in thymomas. However, among the thymoma subtypes, no statistically significant difference was found.
Full table
Patients with B2 and B3 thymomas and TC were significantly younger than patients with A thymomas (P=0.0008, P=0.0101 and P=0.0095, respectively).
No statistically significant differences could be detected between thymoma and TC (P=0.8520) on tumor size.
In our population, more than 95% of patients with A, AB, B1 thymomas showed Masaoka stage I or II. Higher stages increased from B2 through B3 thymomas to TCs.
The difference between resection rate in TCs and all thymomas was highly significant (P<0.0001). Between thymoma subtypes, rates of incomplete resections were only slightly significant (P=0.0071).
Among thymoma patients (n=183), 23.7% had MG; it occurred most frequently in B2 thymomas. The difference between thymoma and TC patients (only 2%) was highly significant (P=0.0001). More than that, among B2 thymomas patients MG was significantly more common compared to A-AB, B1 or B3 types (P=0.0401, P=0.0037, P=0.0142 or P=0.0396, respectively). The only one MG-associated TC (squamous cell carcinoma) showed a minor B3 component. Pure TCs were not associated with MG.
Adjuvant and neoadjuvant treatment of thymic epithelial tumors (TETs)
Due to lack of standardized treatment protocols, therapies for TETs were diverse. Postoperative radiotherapy was used irrespective of stage and resection status in 57% of thymomas, but in only 31% of TCs (P=0.0006). By contrast, neoadjuvant protocols (with or without subsequent adjuvant therapy) were only applied in patients with B1 (2/18), B2 (2/46) and B3 (2/33) thymomas and TCs (8/58).
Follow-up in terms of relapses, metastasis and survival
No patient with A or AB thymoma died of tumor, 1 AB thymoma patient showed relapse. Among 18 B1 thymoma patients, 7 showed relapse or metastasis (39%) and 1 died of tumor. Fourteen of 46 B2 thymomas relapsed or metastasized (30%) and 10 (22%) were the cause of death. Among 33 B3 thymomas, 11 (33%) relapsed or metastasized and 4 (12%) caused death. Relapse/metastasis (67%) and tumor-related death rates (62%) were highest in TC patients. The association between progression and histological subtype as well as between outcome and histotype are highly significant (each P<0.0001). Tumor-related death was most common in advanced stage (Masaoka III or IV) TETs and B2, B3 thymoma and TC.
Detailed survival analysis
Survival and histology
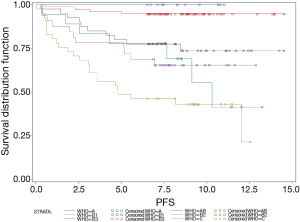
The 5-year OS of patients with A, AB, B1, B2, B3 thymomas and TCs were 100%, 100%, 94%, 80%, 94%, and 45% respectively. In order to assess PFS, data of only 217 patients was available because of 24 missing values regarding PFS. Thus, the 5-year PFS were 100%, 96%, 78%, 80%, 78% and 39% (Figure 1). OS and PFS were significantly different between thymoma and TC patients (each P<0.0001). By contrast, neither the differences in OS nor PFS, were significant between B1, B2 and B3 thymoma patients (P=0.3161 and P=0.4872, respectively). Also, PFS of A and AB thymoma patients showed no significant differences (P=0.4825 and P=0.4158).
Survival and Masaoka stage
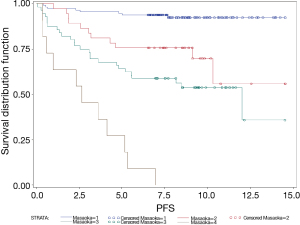
The 5-year OS of Masaoka stage I, II, III and IV patients were 96%; 89%; 59% and 50%, respectively (P<00001). The 5-year PFS of patients with Masaoka stage I, II, III and IV were 95%; 76%; 64% and 27%, respectively (P<0.0001) (Figure 2).
Survival and resection status
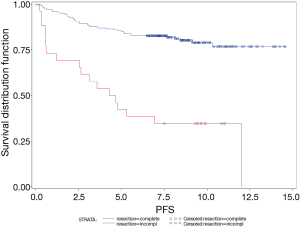
The 5-year OS of patients with complete and incomplete resection were 87% and 46%, respectively (P<0.0001). The 5-year PFS of patients with complete and incomplete resection were 85% and 42%, respectively (P<0.0001) (Figure 3).
Univariate and multivariate analysis of risk factors in terms of survival
By univariate analysis using Log-rank tests, gender (P=0.0413), tumor size (P=0.0003), MG status (P=0.0518), histotype (P<0.0001), Masaoka stage (P<0.0001), resection status (P<0.0001) and treatment (P<0.0001) were associated with OS, while age was not (P=0.7801). Female gender, small tumor size and presence of MG were favorable prognostic markers. Patients who received postoperative chemotherapy, radiotherapy combined with chemotherapy or neoadjuvant treatment had better OS than patients without postoperative intervention or surgery followed only by radiotherapy.
Multivariate Cox regression analysis showed only histotype, Masaoka stage and treatment (each P<0.0001) were independent prognostic indicators of OS after adjustment for gender, age, tumor size, MG status and resection status. OS of patients with postoperative chemotherapy (either with or without neoadjuvant treatment) was significantly better than OS of patient who received radiotherapy after surgery (P=0.0003).
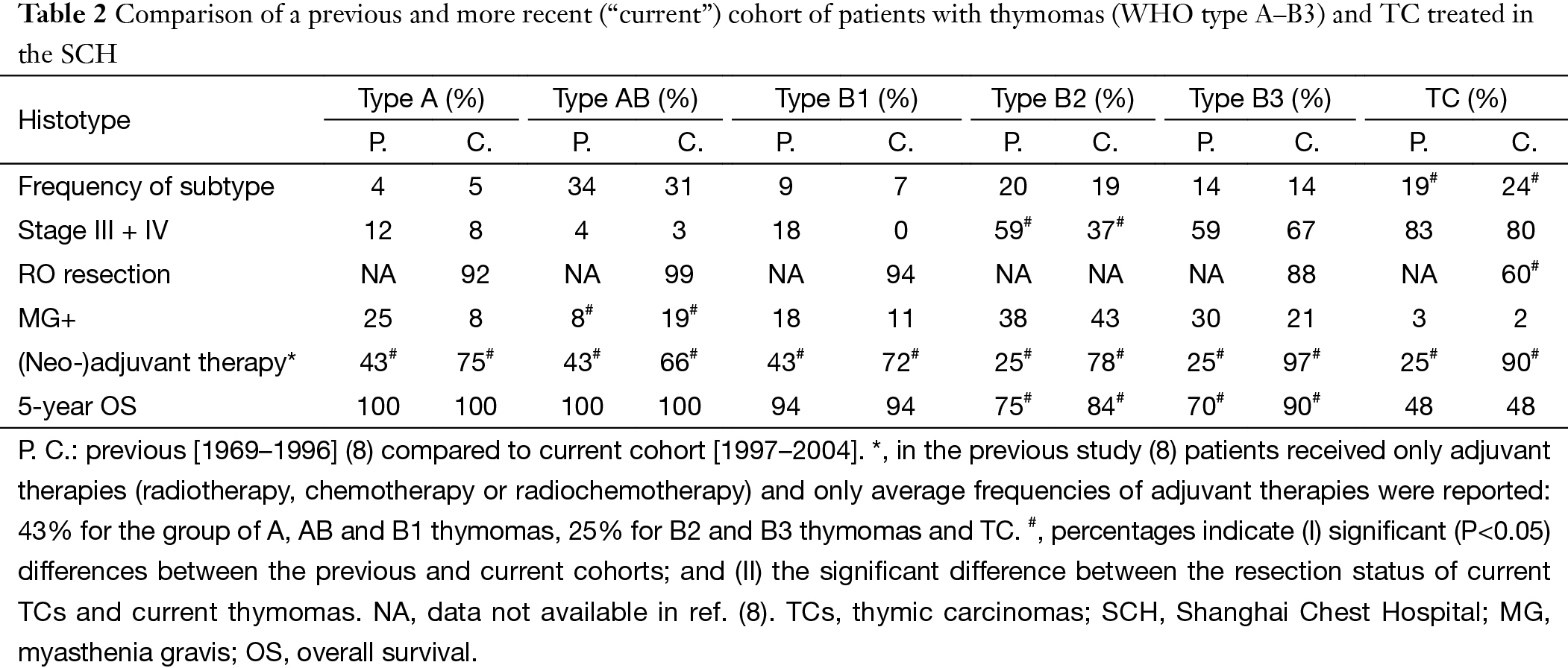
The comparison of some clinicopathological data of two different periods [previous, 1969–1996 (8) vs. current cohort, 1997–2004] in the same institution was listed in Table 2. We could conclude epidemiological and pathological findings were almost unchanged, as was the poor prognosis of patients with TC. By contrast, 5-year OS of B2 and B3 thymoma patients improved substantially.
Full table
Discussion
In 2002, colleagues of SCH reported 200 patients with TETs treated between 1969 and 1996 (8). To address whether characteristics and survival of TET patients changed since then, we studied a consecutive, non-overlapping cohort of 241 patients with thymoma and TC treated in SCH between 1997 and 2004.
Spectrum of thymic epithelial tumors (TETs)
Unlike some reports which found type A and B1 thymomas were highly prevalent (3,10), WHO AB, B2, B3 thymomas and TCs were the predominant tumors in the current and previous study from SCH (8) as well as in most other series (9,11-13). This is at variance with other.
Gender distribution
Male predominance among TC patients (P=0.00) was also found by the previous study from SCH (8) and most other series (14). The thymoma subtypes in the current and previous study from SCH (8) and elsewhere (9) showed no gender difference.
Myasthenia gravis (MG) association
The difference in prevalence of MG in series from different centers likely reflects recruitment bias. Since SCH is a specialized hospital and do not have a neurology department, prevalence of MG among TET patients has been low in the previous (15%) (8) and current series (18.7%). Nevertheless, the higher prevalence of MG in type B2 and B3 compared with other subtypes echoes findings from virtually all other published series (8,9,13-15). Presence of MG was associated with better survival in univariate analysis. This result is opposite to that of the previous SCH cohort (8) but similar to that of Strobel (13). Improved diagnosis and management of MG during recent decades and earlier detection of MG+ thymomas might have contributed to this effect (13).
Histotype and tumor stage
The majority of patients with A and AB thymomas were in Masaoka stage I or II, while Masaoka stages III and IV were seen mainly in B2 and B3 thymomas and TCs. This association was observed by most researchers previously (8,10-18). Stage III and IV TC were as frequent in the current (80%) as in the historical cohort (83%) (8), ruling out the potential selection bias and assuring the consistently poor prognosis of TC. By contrast, there were more stage I (50%) and less stage III (28%) B2 thymomas in the current series than the historical cohort (stage I: 28% and III: 49%) (8) suggesting earlier tumor detection. The latter could be due to the particularly high association of B2 thymoma with MG which might have led to earlier detection of the disease (13).
Survival related parameters
Histotype and survival
Like many previous studies, we observed an association between histological subtype and survival (6,8,14,16,19). The well-known (8,13) excellent prognosis of A and AB thymomas was confirmed. Nevertheless, they should be considered as tumors of low malignant potential, since lethal A and AB thymomas have been reported (6,7,11,17,18,20). OS was significantly better in thymomas than TCs, while differences between B1, B2 and B3 thymomas were not significant. The latter finding is different from that of the previous study from the SCH (8). Furthermore, OS of recent B2 and B3 thymoma patients was better (80% and 94%, respectively) than that of their historic counterparts (75% and 70%, respectively) (8,21). Both observations could be related to the higher number of low stage B2 thymomas in the recent cohort and broader use of (neo-)adjuvant therapies in B2 and B3 thymomas (see below). B2, B3 thymomas and TCs were clearly malignant, while B1 thymomas behaved in an intermediate way between type A/AB and B2/B3 thymomas.
Tumor stage and survival
As in most literature (10,12,14,16-18,22), OS of previous (8,21) and current patients with stage III and IV disease was significantly worse than OS of patients with stage I disease, while there was no significant difference between stage I and II and between stage III and IV tumors. Among stage I and II tumors, OS of TCs was significantly worse than that of thymomas, while there was no difference among thymoma subtypes. Among stage III and IV tumors, OS of B3 thymoma patients was still better than of TC.
Similar to the previous series (8), OS of current B2 thymoma patients was different in lower-stage (I, II; 1 of 29 patients died) and advanced stage tumors (III, IV; 9 of 17 patients died) (P=0.00). By contrast, and against a background of improved OS, this difference was not significant in current B3 thymoma patients (1 of 11 stage I/II versus 4 of 22 stage III/IV patients died). OS of TCs patients were not associated with tumor stage, however, even stage I and II TC patients showed poor outcome. This reflected unique biological features of TCs, as already suggested by genetic (23,24), immunohistochemical (19) and functional studies (25).
Tumor size and survival
Multivariate analysis suggested that tumor size was not an independent prognostic factor for OS. This result appears different from that of Wright et al. (26), who described an association between size and recurrence. Tumor diameters were not recorded in the previous SCH study (8), therefore we do not know whether TETs in the current series were detected at a smaller size. Unspecific symptoms or tumor markers herald only advanced tumors, while specific markers (e.g., autoantibodies) may help identify thymomas but not TCs (13,27).
Resection status and survival
Resection status was not mentioned in the previous paper (8), but reported in subsequent paper (21). Complete resection has been reported as prognostic factor of TETs (9,13,16,21) as confirmed here by univariate but not multivariate analysis. However, when (neo-)adjuvant treatment was omitted from the Cox regression analysis, resection status became a significant variable (P=0.025), suggesting a correlation between positive margins and use of (neo-)adjuvant interventions. Complete resection was associated with improved OS in patients with B2 and B3 thymomas and TCs (P=0.0366; P=0.0863; and P=0.0196, respectively). Only after complete was OS of B2 or B3 thymomas statistically different from OS of TCs (P<0.0001 and P=0.0848, respectively).
Adjuvant and neoadjuvant treatment and survival
Compared to surgery as the only treatment, postoperative combined chemoradiation, adjuvant chemotherapy alone, and neoadjuvant approaches but not postoperative radiation alone (see below) were associated with improved OS in TETs patients. However, the current and historical patients with A, AB and B1 thymomas showed almost 100% OS irrespective of adjuvant therapies. For unknown reasons, adjuvant therapies were used more frequently in current than historical A, AB and B1 thymoma patients. In fact, it has already been widely accepted that adjuvant therapies might be of no benefit in stage I and II thymomas (28). Therefore, almost all current A, AB and B1 thymoma patients who received adjuvant therapies were apparently overtreated.
The insignificant association between postoperative radiotherapy and OS might also be due to the fact that 62 of 122 patients with postoperative radiotherapy had A, AB and B1 thymomas and excellent survival irrespective of adjuvant treatment. In the other 60 B2, B3 thymomas or TC patients who received radiation, OS was not significantly different from OS of the 17 patients treated by surgery alone. But it is difficult to reach definite conclusion due to the small case number. Prospective clinical trials are needed to define the role of adjuvant radiation in stage III thymoma and TC patients.
The significant association between (neo-)adjuvant therapies and improved OS was mainly attributable to better OS in B2 and B3 thymomas. This finding confirms the association between the use of (neo-)adjuvant therapies and improved OS of B2, B3 thymoma patients in our historical cohort (8). These identical observations in two independent cohorts are in line with the finding that broader use of (neo-)adjuvant therapy in recent (97%) compared to historical (~25%) but otherwise similar B3 thymomas from the SCH was associated with better OS. Similar conclusion in B2 thymomas is less safe, since their improved OS was associated not only with intensified (neo-)adjuvant therapy but also with more stage I and II tumors. The hypothetical favorable effect of (neo-)adjuvant therapy would also explain the surprisingly better 5-year OS of current B3 (OS 90%) compared to current B2 thymoma patients (84%): 97% of the former received (neo-)adjuvant treatment, compared to 78.2% of the latter. Obviously, we cannot exclude that better anesthesia, surgery and postoperative care contributed to the better outcome of current B2 and B3 thymoma patients. However, if these factors were of critical relevance, one would expect a similar improvement of OS in the current patients with TCs—which was not the case: in spite of much broader use of (neo-)adjuvant therapies, the 5-year OS of recent TC patients remained at the historical level of 48%. Therefore, we cautiously prefer the interpretation that recent B3 (and maybe B2) thymoma patients profited from the broader use of (neo-)adjuvant approaches, while there was no benefit for TC patients. Consequently, the effect of (neo-)adjuvant therapies in stage III and IV thymomas needs to be confirmed by prospective randomized trials. Considering the poor effects of intensified adjuvant treatments and infrequent use of neo-adjuvant therapies in the current (14%) and historical (0%) cohorts, neo-adjuvant or other innovative approach (e.g., target therapy) may be more preferable in TC patients.
In summary, we found that prognosis of stage III and IV, B2 and B3 thymomas at a single institution improved during the last decade, in parallel with the broader use of adjuvant chemotherapy or combined chemo-radiation. By contrast, the poor outcome in TCs remained unaltered in spite of the same broader use of adjuvant therapies, suggesting that neo-adjuvant and innovative strategies should be tested in these patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Marchevsky AM, Gupta R, McKenna RJ, et al. Evidence-based pathology and the pathologic evaluation of thymomas: the World Health Organization classification can be simplified into only 3 categories other than thymic carcinoma. Cancer 2008;112:2780-8. [Crossref] [PubMed]
- Marino M, Müller-Hermelink HK. Thymoma and thymic carcinoma. Relation of thymoma epithelial cells to the cortical and medullary differentiation of thymus. Virchows Arch A Pathol Anat Histopathol 1985;407:119-49. [Crossref] [PubMed]
- Rosai J, Sobin LH. Histological Typing of Tumors of the Thymus. 2nd ed. Berlin: Springer, 1999.
- Travis WD, Brambilla E, Müller-Hermerlink Hk., et al. WHO Classification of tumors of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press, 2004.
- Suster S, Moran CA. Histologic classification of thymoma: the World Health Organization and beyond. Hematol Oncol Clin North Am 2008;22:381-92. [Crossref] [PubMed]
- Rieker RJ, Hoegel J, Morresi-Hauf A, et al. Histologic classification of thymic epithelial tumors: comparison of established classification schemes. Int J Cancer 2002;98:900-6. [Crossref] [PubMed]
- Chalabreysse L, Roy P, Cordier JF, et al. Correlation of the WHO schema for the classification of thymic epithelial neoplasms with prognosis: a retrospective study of 90 tumors. Am J Surg Pathol 2002;26:1605-11. [Crossref] [PubMed]
- Chen G, Marx A, Chen WH, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95:420-9. [Crossref] [PubMed]
- Detterbeck FC. Clinical value of the WHO classification system of thymoma. Ann Thorac Surg 2006;81:2328-34. [Crossref] [PubMed]
- Verghese ET, den Bakker MA, Campbell A, et al. Interobserver variation in the classification of thymic tumours--a multicentre study using the WHO classification system. Histopathology 2008;53:218-23. [Crossref] [PubMed]
- Kim DJ, Yang WI, Choi SS, et al. Prognostic and clinical relevance of the World Health Organization schema for the classification of thymic epithelial tumors: a clinicopathologic study of 108 patients and literature review. Chest 2005;127:755-61. [Crossref] [PubMed]
- Park MS, Chung KY, Kim KD, et al. Prognosis of thymic epithelial tumors according to the new World Health Organization histologic classification. Ann Thorac Surg 2004;78:992-7; discussion 997-8. [Crossref] [PubMed]
- Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501-9. [Crossref] [PubMed]
- Kondo K, Yoshizawa K, Tsuyuguchi M, et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg 2004;77:1183-8. [Crossref] [PubMed]
- Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624-32. [Crossref] [PubMed]
- Nakagawa K, Asamura H, Matsuno Y, et al. Thymoma: a clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg 2003;126:1134-40. [Crossref] [PubMed]
- Rea F, Marulli G, Girardi R, et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg 2004;26:412-8. [Crossref] [PubMed]
- Rena O, Papalia E, Maggi G, et al. World Health Organization histologic classification: an independent prognostic factor in resected thymomas. Lung Cancer 2005;50:59-66. [Crossref] [PubMed]
- Marx A, Rieker R, Toker A, et al. Thymic carcinoma: is it a separate entity? From molecular to clinical evidence. Thorac Surg Clin 2011;21:25-31. v-vi. [Crossref] [PubMed]
- Jain RK, Mehta RJ, Henley JD, et al. WHO types A and AB thymomas: not always benign. Mod Pathol 2010;23:1641-9. [Crossref] [PubMed]
- Fang W, Chen W, Chen G, et al. Surgical management of thymic epithelial tumors: a retrospective review of 204 cases. Ann Thorac Surg 2005;80:2002-7. [Crossref] [PubMed]
- Masaoka A. Staging system of thymoma. J Thorac Oncol 2010;5:S304-12. [Crossref] [PubMed]
- Zettl A, Ströbel P, Wagner K, et al. Recurrent genetic aberrations in thymoma and thymic carcinoma. Am J Pathol 2000;157:257-66. [Crossref] [PubMed]
- Girard N, Shen R, Guo T, et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin Cancer Res 2009;15:6790-9. [Crossref] [PubMed]
- Kaira K, Murakami H, Serizawa M, et al. MUC1 expression in thymic epithelial tumors: MUC1 may be useful marker as differential diagnosis between type B3 thymoma and thymic carcinoma. Virchows Arch 2011;458:615-20. [Crossref] [PubMed]
- Wright CD, Wain JC, Wong DR, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg 2005;130:1413-21. [Crossref] [PubMed]
- Marx A, Willcox N, Leite MI, et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity 2010;43:413-27. [Crossref] [PubMed]
- Detterbeck FC, Parsons AM. Management of stage I and II thymoma. Thorac Surg Clin 2011;21:59-67. vi-vii. [Crossref] [PubMed]


