Clinical characteristics of idiopathic pulmonary fibrosis patients according to their smoking status
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common and relentless fibrotic lung disease characterized by both exertional dyspnea, non-productive cough, and often has the presence of honeycombing observed via high-resolution computed tomography (HRCT) (1-3), and a usual interstitial pneumonia (UIP) pattern defines the radiographic and histologic presentation. However, not all patients with IPF have honeycombing. Therefore, IPF patients often show possible UIP pattern of HRCT (4). The median survival of patients with IPF is approximately 3 years (5) and the commonest causes of death are acute exacerbation (AE) and progressive respiratory failure (6,7). The clinical course of IPF patients varies from stable to AE (8,9). Therefore, predicting the survival of IPF patients is particularly challenging for physicians.
Majority of IPF patients are elderly males with a history of smoking. However, we often encounter approximately 30% never-smoking IPF patients (4,10). There are relatively few reports with detailed information regarding never-smoking IPF patients. Therefore, the aim of this study was to clarify the clinical characteristics of both never-smoking patients and smoking IPF patients who were followed-up at our hospital.
Methods
We retrospectively retrieved medical records of IPF patients who underwent pulmonary function tests and chest HRCT scans from July 1, 2008 to June 30, 2013 based on ICD-9 codes at our hospital, and we evaluated chest HRCT patterns based on the 2011 International IPF guidelines (11).
Patient characteristics at diagnosis included age, gender, smoking history, passive smoking history, family history of interstitial lung disease, modified Medical Research Council (mMRC) dyspnea scale (12), dyspnea duration, presence of pulmonary hypertension (PH), IPF treatments and survival. We also repeated evaluations of mMRC scores a year later. The laboratory findings of white blood cell (WBC) count, lactate dehydrogenase (LDH), and Krebs von den Lungen-6 (KL-6) values at diagnosis were reviewed. Physiological findings included percent predicted forced expiratory volume (1 second) (%FEV1), forced vital capacity (FVC), percent predicted FVC (%FVC), total lung capacity (TLC), percent predicted TLC (%TLC), diffusing capacity of the lung for carbon monoxide (DLco), percent predicted DLco (%DLco), DLco/alveolar volume (KCO), and percent predicted KCO (%KCO). DLco were performed with a single breath method. All pulmonary function data were also obtained based on ATS guideline of diagnosis (13). Each patient underwent annual echocardiographic examinations for evaluation of PH. PH was defined as estimated systolic pressure over 40 mmHg based on tricuspid valve regurgitation (TR) velocity.
Chest CT was obtained with 1.5-mm-thick axial sections at 1-cm intervals throughout the entire thorax in the inspiratory phase. No oral or intravenous contrast material was administered. We chose three levels for imaging scoring. These levels were (aortic arch, carina, and 1 cm above the right diaphragm). We evaluated reticulation, traction bronchiectasis, honeycombing, and emphysema. Reticulation was defined as interlace lines within secondary lobule.
In terms of scoring, reticulation was defined as follows: 0, none; 1, involvement >25% of each zone; 2, 25%–50% of each zone; and 3, >50% of each zone. Traction bronchiectasis was defined as abnormal bronchial dilatation with irregular bronchial walls. Traction bronchiectasis was scored as follows: 0, none; 1, bronchial dilatation involving bronchi distal to the fifth generation; 2, bronchial dilatation involving fourth-generation bronchi; and 3, bronchial dilatation involving third-generation bronchi. Honeycombing was defined as a cluster of relatively thick-walled (1–3 mm) cysts, 3–10 mm in diameter, with or multiple cysts that shared walls and a single layer of clustered subpleural cysts located in the periphery of the lung, thereby ensuring that excluding paraseptal emphysema is not present (14). Honeycombing was scored as follows: 0, none; 1, involvement >25% of each zone; 2, 25%–50% of each zone; and 3, >50% of each zone (15,16). Emphysema was defined as low attenuation area with a CT value of less than −960 hounsfield unit (HU) without definite walls. Emphysema was scored as follows: 0, none; 1, less than 10% involvement; and 2, over 10% involvement. These scores were assessed in each of the six lung zones and total score was calculated. Average score was defined as total score divided into six. These CT data were evaluated at the initial stage.
Regarding physiology, we also calculated two validated composite measures of pulmonary physiology that predict disease progression and mortality. We evaluated gender, age, and physiology (GAP) staging system using %FVC and %DLco values (17). We also calculated the composite physiologic index (CPI) according to the following formula (18): CPI =91−(0.65×%DLco)−(0.53×%FVC)+(0.34×%FEV1).
Follow-up period was defined from the diagnosis date based on pulmonary function test until the death or cutoff date. Cutoff date was defined as June 30, 2014 because we wished to obtain 1-year mMRC for final patient.
Patient approval or informed consent was waived because the Institutional Review Board of Okinawa Chubu Hospital determined that this study was retrospective review of patient records and physiology and images. AE was defined as a sudden aggravation of dyspnea within 30 days with new bilateral infiltration accompanying known IPF or evidence of honeycombing on HRCT of the chest (19). Patient records/information was anonymized and de-identified prior to analysis in this study. The Institutional Review Board of Okinawa Chubu Hospital approved this retrospective study.
Statistical analysis
Continuous variables are presented as medians, and categorical variables are presented as percentages. The Chi-square and Fisher’s exact tests were used to analyze categorical data, and the unpaired t-test was used for continuous data. Cox regression analysis was used to identify significant variables predictive of mortality.
We performed univariate analysis for possible predictors based on previous study about IPF. (11) Threshold for the candidates of predictor of AE and mortality was P<0.1. After selecting the candidates, we performed stepwise approach of multivariable analysis for the predictors of AE and mortality. The Kaplan-Meier survival curves and the log-rank test were used to evaluate survival. The level of statistical significance was set at P<0.05. All analyses were performed using Stata Data Analysis and Statistical Software STATA version 11.0; (Stata Corp., College Station, TX, USA).
Results
Patient characteristics
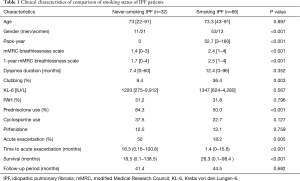
During the observation period, we identified 32 never-smoking IPF patients who were followed-up at our hospital. In addition, we reviewed 66 consecutive smoking IPF patients. The clinical characteristics of these IPF patients are shown in (Table 1). The follow-up period was not significantly different (41 vs. 44 months).
Full table
The never-smoking patient cohort included 11 men and 21 women aged approximately 73 [22–91] years. Of these, three patients had a family history of interstitial lung disease and eight reported a history of exposure to passive smoking.
Clinical symptom: the mean mMRC dyspnea scale score and dyspnea duration of never-smoking and smoking group were 1.4 (0–3) vs. 2.4 [1–4] months, P=0.0002, and 7.4 (0–60) vs. 12.4 (0–96) months, P=0.352, respectively.
Physical findings: never-smoking IPF patients had clubbing less often (9.4% vs. 36.4%, P=0.003) than the smoking IPF patients.
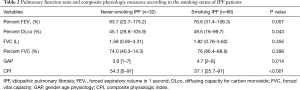
Pulmonary function: with regard to pulmonary function, although %FEV1 was significantly higher in non-smokers than in smokers, %DLCO was lower in non-smokers than in smokers (Table 2). In addition, the mean GAP and CPI of never-smoking IPF and smoking IPF groups were 3.9 [1–7] vs. 4.7 [2–8], P=0.011 and 54.3 [0–91] vs. 37.1 [25.7–91], respectively. At the diagnosis stage, never-smoking IPF patients showed similar pulmonary dysfunction compared with smoking IPF patients. However, combined index such as CPI showed more severe functional impairment in never smoking IPF patients.
Full table
Comorbidities: in terms of PH, there is no significant difference between the groups. Eight patients (25%) showed PH during the observation period. On the other hand, 12 smoking IPF patients (18%) had PH. Among never-smoking IPF patients, no one satisfied the criteria for connective tissue diseases. Five patients were positive for anti-nuclear antibody or rheumatoid factor. Two patients showed sicca symptoms, such as dry mouth.
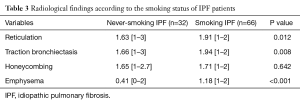
Radiological findings: among never smoking IPF patients, chest HRCT findings showed that 22 patients had definite UIP patterns and 10 patients had possible UIP patterns based on the 2011 International IPF guidelines. Among the possible UIP pattern patients, seven patients underwent surgical lung biopsy (SLB) and showed definite pathological UIP patterns. The remaining three patients were diagnosed with IPF based on multi-disciplinary discussions. These three patients had clubbing and HRCT showed lower lobe predominant reticular opacity with definite traction bronchiectasis. In addition, these patients showed progressive decline of %FVC over three months. Compared with smoking IPF patients, never-smoking IPF patients revealed less traction bronchiectasis and emphysema (1.66 vs. 1.94, P=0.008, and 0.41 vs. 1.18, P<0.001, respectively) (Table 3). In smoking IPF patients, 10.6% underwent SLB for definite diagnosis and 68% underwent multi-disciplinary discussion. The remaining 32% patients were diagnosed by discussion of four chest physicians. In majority of these patients without SLB, the HRCT pattern showed definite UIP pattern.
Full table
Treatment: regarding treatment, 14 patients (43.8%) received prednisolone monotherapy, 12 patients (37.5%) took prednisolone and cyclosporine combination therapy, 4 patients (12.5%) received pirfenidone monotherapy in never-smoking patients. Two patients were followed-up without treatment. About 84% of the never smoking IPF patients received prednisolone. On the other hand, only half of the patients took prednisolone and 15 patients (22.7%) received cyclosporine in smoking IPF patients. No patients underwent lung transplantation.
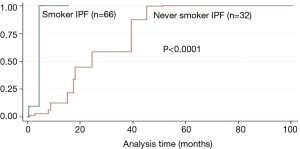
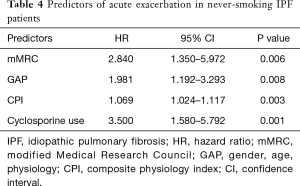
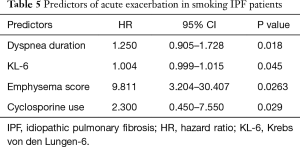
AE: 50% and 18% of the never-smoking and smoking patients, respectively, developed AE during the 18.5 months. The median period from diagnosis to AE of never-smoking and smoking patients was 16.3 (0.16–100.8) and 1.4 (0–15.6) months, respectively, P<0.001. After the adjustment of CPI and prednisolone use, never-smoking IPF patients tended to show AE more often and late than that of smoking IPF patients (Figure 1), and 68.8% of AE developed during winter and early spring. The predictors of AE in never-smoking IPF patients included mMRC breathlessness scale [hazard ratio (HR), 2.84; P=0.006), GAP (HR, 1.981; P=0.008), CPI (HR, 1.069; P=0.003), and cyclosporine use (HR, 3.500; P=0.001) after adjustment of age and dyspnea duration (Table 4). In smoking IPF patients, the strong predictors of AE were dyspnea duration (HR, 1.25; P=0.018), KL-6 (HR, 1.00; P=0.045), emphysema score of chest HRCT (HR, 9.81; P=0.026), and cyclosporine use (HR, 2.300; P=0.029) (Table 5). The ROC curve of KL-6 for AE showed that over 1,200 IU/L correctly classified of the 74% of smoking IPF patients and the area under ROC curve was 0.691.
Full table
Full table
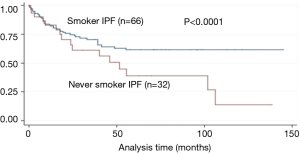
Survival: the median survival period of never-smoking and smoking IPF patients was 18.5 (0.1–38) and 26.3 (0.1–98) months, respectively, P<0.001] (Table 1). After the adjustment of baseline CPI, never-smoking IPF patients showed worse prognosis than that of smoking IPF patients (P<0.001) (Figure 2). The results of the Cox proportional hazard model showed that the 1-year mMRC breathlessness scale (HR, 3.24; P=0.001) and GAP score (HR, 1.59; P=0.029) were strong predictors of mortality in never smoking IPF patients (Table 6).

Full table
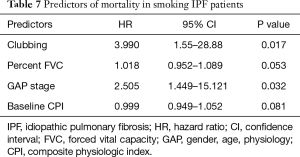
On the other hand, clubbing (HR, 3.990; P=0.017) and GAP stage (HR, 2.505; P=0.032) were strong predictors of mortality in smoking IPF patients (Table 7). Among the deceased patients, 30.8% patients died from pneumonia, 25.6% from AE, 20.5% from progressive respiratory failure and 11.1% from lung cancer.
Full table
Discussion
Here we described the comparison of clinical characteristics of never-smoking and smoking IPF patients in our hospital. Compared with typical IPF patients, our cohort had more women and patients with a greater family history of interstitial lung disease. Steele, et al. described approximately 309 familial interstitial pneumonia (FIP) patients and they found that patient age, male sex, and smoking history were important risk factors of FIP (20). Therefore, individual susceptibility to fibrosis may partly explain the development of IPF (21-23). In addition, a quarter of these patients had a history of exposure to passive smoking. Passive smoking may contribute to emphysematous change in never-smoking IPF patients. Also, the members of our patient cohort had no specific occupational exposure history. Further studies are required to examine these issues. In our cohort, none of the never-smoking IPF patients satisfied the criteria of connective tissue disease. Regarding age and dyspnea duration, there was no significant difference between both never-smoking IPF patients and smoking IPF patients. Therefore, there is no lead time bias in our cohort. A majority of patients in our cohort had possible UIP patterns of HRCT, indicating a greater risk for the development of pathological UIP. These findings were in accordance with those of a previous report (24). We need future studies of patients with surgical biopsy to identify the criteria for a definite diagnosis of the possible UIP (25). When evaluating potential predictors of AE in our patients, we found that the mMRC score, GAP score, and CPI were useful parameters in never-smoking IPF patients. Song et al. (26) reported that never smoking and reduced FVC were risk factors for AE of IPF. Our never-smoking cohort had high incidence of AE in the long term than smoking IPF patients. We could not identify the reason of this event. In clinical course, never-smoking IPF patients tended to have more progressive exertional dyspnea than that of non-AE patients. Therefore, among the heterogeneous IPF patients, never-smoking IPF patients may be able to take sub-acute progression of disease activity. In addition, majority of AE appeared during winter. Viral infection and environment may contribute to it. Therefore, we should monitor the symptoms, physiology, and seasonal change cautiously. On the other hand, dyspnea duration, KL-6, and HRCT emphysema score were strong predictors of AE in smoking IPF patients. Our laboratory findings of smoking IPF patients revealed a relatively high mean KL-6 value which may signify a greater risk for fibrosis (27-29). In addition, serum KL-6 was the predictor of smoking IPF patients. Therefore, an elevation of KL-6 such as over 1,200 was an alarming sign for possible AE in our cohort. Therefore, KL-6 and smoking impact were reflected in the prediction of AE in typical IPF patients. This result was in accordance with our previous report on combined pulmonary fibrosis and emphysema patients (30). Thus, besides fibrosis, we should monitor both KL-6 and the extent of emphysema in smoking IPF patients. Based on our result, high value of KL-6 indicates early AE in smoking IPF patients. Severe alveolar epithelial injury may cause early AE. In addition, emphysema has a strong relationship with AE in smoking IPF patients; there is a possibility of a relationship between capillary damage and AE in smoking IPF patients. The etiology of less development of AE in smokers remains unknown. For both never-smoking and smoking IPF patients, immunosuppressants use such as cyclosporine is a predictor of AE (Tables 4,5). This is accordance with the PANTHER trial (31). We usually used prednisolone and other immunosuppressant therapy for progressive disease before the anti-fibrotic agent era. Because progression of disease increases with increase in cyclosporine use, intensive therapy is associated with the development of AE or infection in IPF patients.
For physicians, it is important to choose which patients to treat and how to monitor or predict the course of progressive diseases, such as IPF. In addition, when using simple parameters, it is possible to create an applicable staging system for hospitals worldwide. The clinical courses of the IPF patients in this study widely varied; therefore, disease activity should be carefully monitored (32). From a clinical point of view, cough has been proposed as a prognostic indicator of IPF (33,34). Never-smoking IPF patients showed poor survival after adjustment of baseline CPI. Analysis of the predictors of mortality in our cohort identified both 1-year mMRC and GAP score as strong predictors. We believe that our findings regarding changes in mMRC should be considered in the breathlessness scale and mortality in never-smoking IPF patients. Du Bois et al. (35) reported a positive correlation between changes in dyspnea and %FVC. The most recent idiopathic interstitial pneumonia guidelines propose consideration of disease behavior (36). Serial monitoring of trends in major clinical symptoms is crucial to the management of this progressive disease. Also, finger clubbing and GAP stage were useful predictors of mortality in smoking IPF patients. Both detailed physical examination and severity of physiology is quite important for typical IPF patients. Regarding smoking status and physiology, previous articles reported that smoking IPF patients had less severe pulmonary dysfunction because of smoker healthier effect (37,38).
Finally, in terms of treatment, many patients underwent PSL-based therapy, of which 50% developed AE during the observational period. AE following IPF is a common cause of death (19,39). After the adjustment of prednisolone use, never-smoking IPF patients had AE more often compared with that of smoking IPF patients. Pirfenidone has been previously reported to be effective for prevention of AE in IPF patients (40-42). In addition, pirfenidone was shown to reduce disease progression, while maintaining exercise tolerance and promoting progression-free survival (10). Pirfenidone is a promising agent for the prevention of AE in never-smoking IPF patients in the future. Moreover, we recently published staging-based management to monitor AE in IPF patients (43).
There were several limitations to this study. First, this was a retrospective study; therefore, some clinical data were missing. Second, this study was conducted in a single center, and our hospital is a referral center for IPF. Therefore, there could be a selection bias. Our results of this study may not be applicable to all never-smoking IPF patients. However, the epidemiological and clinical characteristics of our cohort were comparable to those cited in previous reports. Third, we did not evaluate the detailed genotype for MUC5b, TERT/TERC and telomere length. Future studies will be needed to elucidate the genetic background of never-smoking IPF patients. Fourth, we were unable to completely evaluate changes in dyspnea grade and lung function.
In conclusion, we clarified the clinical characteristics of IPF in never-smoking patients through comparison with smoking IPF patients. Never-smoking patients develop AE more often and 1-year mMRC dyspnea scale was an important predictor of mortality. In smoking IPF patients, clubbing and physiology were useful predictors of mortality, and HRCT emphysema extent was a crucial predictor of AE. Combined evaluation of clinical symptom trends and physiology is important to elucidate disease behavior and predict mortality in never-smoking IPF patients. Future multi-center studies are needed to validate our results for never-smoking IPF patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lynch DA, Godwin JD, Safrin S, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med 2005;172:488-93. [Crossref] [PubMed]
- Nishimura K, Kitaichi M, Izumi T, et al. Usual interstitial pneumonia: histologic correlation with high-resolution CT. Radiology 1992;182:337-42. [Crossref] [PubMed]
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646-64. [Crossref] [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;157:199-203. [Crossref] [PubMed]
- Fernández Pérez ER, Daniels CE, Schroeder DR, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest 2010;137:129-37. [Crossref] [PubMed]
- Okamoto T, Ichiyasu H, Ichikado K, et al. Clinical analysis of the acute exacerbation in patients with idiopathic pulmonary fibrosis. Nihon Kokyuki Gakkai Zasshi 2006;44:359-67. [PubMed]
- Martinez FJ, Safrin S, Weycker D, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 2005;142:963-7. [Crossref] [PubMed]
- Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431-40. [Crossref] [PubMed]
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083-92. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581-6. [Crossref] [PubMed]
- Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J 2005;26:153-61. [Crossref] [PubMed]
- Watadani T, Sakai F, Johkoh T, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology 2013;266:936-44. [Crossref] [PubMed]
- MacDonald SL, Rubens MB, Hansell DM, et al. Nonspecific interstitial pneumonia and usual interstitial pneumonia: comparative appearancesat and diagnostic accuracy of thin-section CT. Radiology 2001;221:600-5. [Crossref] [PubMed]
- Sumikawa H, Johkoh T, Colby TV, et al. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med 2008;177:433-9. [Crossref] [PubMed]
- Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684-91. [Crossref] [PubMed]
- Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003;167:962-9. [Crossref] [PubMed]
- Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636-43. [Crossref] [PubMed]
- Steele MP, Speer MC, Loyd JE, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med 2005;172:1146-52. [Crossref] [PubMed]
- Hodgson U, Laitinen T, Tukiainen P. Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: evidence of founder effect among multiplex families in Finland. Thorax 2002;57:338-42. [Crossref] [PubMed]
- Marshall RP, Puddicombe A, Cookson WO, et al. Adult familial cryptogenic fibrosing alveolitis in the United Kingdom. Thorax 2000;55:143-6. [Crossref] [PubMed]
- Putman RK, Rosas IO, Hunninghake GM. Genetics and early detection in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2014;189:770-8. [Crossref] [PubMed]
- Raghu G, Lynch D, Godwin JD, et al. Diagnosis of idiopathic pulmonary fibrosis with high-resolution CT in patients with little or no radiological evidence of honeycombing: secondary analysis of a randomised, controlled trial. Lancet Respir Med 2014;2:277-84. [Crossref] [PubMed]
- Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;164:193-6. [Crossref] [PubMed]
- Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356-63. [Crossref] [PubMed]
- Kohno N, Kyoizumi S, Awaya Y, et al. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest 1989;96:68-73. [Crossref] [PubMed]
- Kohno N. Serum marker KL-6/MUC1 for the diagnosis and management of interstitial pneumonitis. J Med Invest 1999;46:151-8. [PubMed]
- Ishikawa N, Hattori N, Yokoyama A, et al. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig 2012;50:3-13. [Crossref] [PubMed]
- Kishaba T, Shimaoka Y, Fukuyama H, et al. A cohort study of mortality predictors and characteristics of patients with combined pulmonary fibrosis and emphysema. BMJ Open 2012;2:e000988. [Crossref] [PubMed]
- Idiopathic Pulmonary Fibrosis Clinical Research Network, Raghu G, Anstrom KJ, et al. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012;366:1968-77. [Crossref] [PubMed]
- Hanamoto S, Ohsuji T, Tsuyuguchi I, et al. Prediction formulas for pulmonary function tests expressed in linear and exponential form for healthy Japanese adults. Nihon Kyobu Shikkan Gakkai Zasshi 1992;30:2051-60. [PubMed]
- Ryerson CJ, Abbritti M, Ley B, et al. Cough predicts prognosis in idiopathic pulmonary fibrosis. Respirology 2011;16:969-75. [Crossref] [PubMed]
- Lechtzin N, Hilliard ME, Horton MR. Validation of the Cough Quality-of-Life Questionnaire in patients with idiopathic pulmonary fibrosis. Chest 2013;143:1745-9. [Crossref] [PubMed]
- du Bois RM, Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med 2011;184:1382-9. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- King TE Jr, Tooze JA, Schwarz MI, et al. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 2001;164:1171-81. [Crossref] [PubMed]
- Antoniou KM, Hansell DM, Rubens MB, et al. Idiopathic pulmonary fibrosis: outcome in relation to smoking status. Am J Respir Crit Care Med 2008;177:190-4. [Crossref] [PubMed]
- Kim DS, Park JH, Park BK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006;27:143-50. [Crossref] [PubMed]
- Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040-7. [Crossref] [PubMed]
- Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:821-9. [Crossref] [PubMed]
- Taniguchi H, Kondoh Y, Ebina M, et al. The clinical significance of 5% change in vital capacity in patients with idiopathic pulmonary fibrosis: extended analysis of the pirfenidone trial. Respir Res 2011;12:93. [Crossref] [PubMed]
- Kishaba T, Tamaki H, Shimaoka Y, et al. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung 2014;192:141-9. [Crossref] [PubMed]