Effect of small body habitus on peri-operative outcomes after robotic-assisted pulmonary lobectomy: retrospective analysis of 208 consecutive cases
Introduction
Mosteller simplified calculation of body surface area (BSA) in metric terms: BSA equals the square root of the product of weight (in kg) times height (in cm) divided by 3,600 (1). BSA is widely used as the biometric unit for normalizing physiologic parameters (cardiac output, left ventricular mass, renal clearance) and for determination of appropriate drug dosages in cancer chemotherapy in individuals of different body size (2). However, BSA is not a reliable indicator of distribution of fat and adipose tissue, which constitute distribution volumes for lipophilic and hydrophilic chemotherapy agents, respectively (3). The importance of BSA in surgery is related to tissue sizes and operative field exposure.
Smaller body habitus has been associated with increased morbidity in cardiothoracic surgery due to limited access to the surgical field and to small caliber vascularity. In carotid artery endarterectomies, shorter height and smaller BSA were associated with greater operative risk of stroke and death (4).
Patients with smaller BSA have smaller pleural cavities, which limit visualization and instrument mobility during video-assisted thoracoscopic surgery (VATS). Robotic-assisted surgery provides the surgeon a handful of advantages, including a 3-dimensional view of the operating field, better control of instrumentation, capacity to reduce hand-related tremors, and flexibility of instruments with seven directions of articulation, making dissection more precise and accurate (5). However, smaller BSA could result in internal and external collisions between the arms of the robotic patient cart. We investigated the effects of BSA on perioperative outcomes related to robotic-assisted VATS lobectomy.
Methods
We retrospective analyzed prospectively collected data from 211 consecutive patients undergoing robotic-assisted pulmonary lobectomy by one surgeon over a 34-month period from September 2010 through May 2013 at our institution. All our patients gave informed consented for our standard surgical procedure, which consisted of fiberoptic bronchoscopy, video-assisted thoracoscopic insertion of tunneled extrapleural intercostal regional analgesia infusion catheter, robotic-assisted video-thoracoscopic wedge resection and/or robotic-assisted video-thoracoscopic (completion) lobectomy, and mediastinal lymph node dissection, with possible thoracotomy.
Three patients were converted to pneumonectomy and were excluded from the study cohort. We investigated the difference in surgical outcomes between group A, BSA ≤1.65 m2, and group B, BSA >1.65 m2. Operative times, estimated blood loss (EBL), conversion rates to open lobectomy, clinically significant perioperative complication rates, chest tube duration, hospital length of stay (LOS), and in-hospital mortality were noted.
Clinically significant intraoperative and postoperative complications were recorded and compared, including pulmonary embolism, respiratory failure, hemothorax, chyle leak, pneumothorax or mucus plug that required intervention, air leaks persisting more than 7 days, aspiration events, atrial fibrillation, cardiovascular events, and death. An extensive literature review was then performed on effects of BSA on perioperative outcomes after thoracotomy and VATS cases.
Mean, standard error of the mean (SEM), and range were used to report continuous and ordinal variables, such as age, BSA, and body mass index (BMI). Median ± SEM was used for descriptive variables, such as operative times, EBL, chest tube duration, and hospital LOS. We used Student’s t-test, χ2, or Fisher’s exact test, as appropriate to compare variables mentioned above. Statistical significance was established at P≤0.05.
This study was conducted in accordance with the amended Declaration of Helsinki as outcomes research for quality assurance as part of our departmental thoracic oncology clinical research database protocol. This protocol was approved by our Scientific Review Committee (MCC #16512) and by our affiliated university Institutional Review Board (IRB #Pro00002678), which waived informed consent for this retrospective study, which is considered as review of existing data. Nevertheless, all patients gave informed consent for our standard surgical procedure, as previously described. Some patients also gave informed consent for any anticipated en bloc chest wall and/or vertebral resection, with possible reconstruction. Through our institutional surgical informed consent, patients gave permission to use surgery-related and tissue-related data for education and research purposes.
Results
Prior to analyzing our cohort, we observed internal and external collisions of the robotic patient cart arms in patients who weighed 60 kg or less. We hypothesized that these observed collisions were due to decreased distances between robotic arms, which was related to smaller pleural cavities and chest wall areas in these patients. Using BSA as surrogate for chest wall area, we hypothesized that perioperative outcomes may differ between patients with small BSA and with larger BSA.
We identified patients who weighed 60 kg and found that their mean BSA was 1.65 m2. We thus divided our cohort of 208 patients into two groups: group A, n=40 patients (BSA =1.25–1.65 m2), and group B, n=168 patients (BSA =1.66–2.86 m2). Our cohort demographics are shown in Table 1. Group A had mean age 68.5±1.5 years (range, 50–85 years); group B had mean age 68.0±0.8 years (range, 29–86 years). Patients with BSA ≤1.65 m2 have a higher proportion of women than do patients with larger BSA, and BMI was significantly greater in patients with BSA >1.65 m2.

Full table
Intraoperative complication rates did not significantly differ between the two BSA groups. Our overall conversion-to-thoracotomy rate was significant higher for group A, 20%, compared to 7.1% for group B (P=0.03), but emergent conversion rates for bleeding were similar, with 2/40 (5.0%) in group A versus 5/168 (3.0%) in group B (P=0.62) (Table 2).

Full table
Major intraoperative outcomes, such as EBL and skin-to-skin operative times, were similar between groups. Group A had median EBL of 150±96 mL compared to 200±24 mL for group B (P=0.37). Median skin-to-skin operative times were 169±16 min for group A and 176±6 min for group B (P=0.34). Both groups had median hospital LOS of 5± SEM days and had no difference in in-hospital mortality (Table 3).

Full table
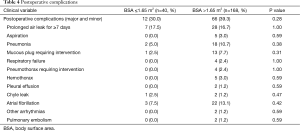
Minor and/or major postoperative complications occurred in 12/40 (30.0%) in group A, compared to 66/168 (39.3%) in group B (P=0.28). The most common postoperative complications in group A were prolonged air leaks for more than 7 days in 7/40 (17.5%), atrial fibrillation in 3/40 (7.5%), and pneumonia in 2/40 (5.0%), while the most common complications in group B were prolonged air leak in 28/168 (16.7%; P=1.0), atrial fibrillation in 22/168 (13.1%; P=0.42), and pneumonia in 18/168 (10.7%; P=0.38) (Table 4).
Full table
Discussion
Our perioperative outcomes are comparable with those previously described for robotic-assisted surgery. Our overall complication rates were 30% and 39% for patients with small BSA and with large BSA, respectively, and our in-hospital mortality rates were 0% and 2%, respectively. Our patients with small BSA and with large BSA had EBL of 150 and 200 mL, respectively, skin-to-skin operative times of 169 and 176 min, respectively, overall conversion-to-thoracotomy rates of 20.0% and 7.1%, respectively, and hospital LOS of 5 and 5 days, respectively.
A systematic review of robotic-assisted VATS lobectomy literature reported overall morbidity rates between 10% and 39%, with perioperative mortality rates between 0% and 3.8% (6). A meta-analysis of currently available robotic literature, including data from 326 patients, showed a pooled average operative time of 215 min, overall conversion rate of 9.4%, mortality rate of 2.1%, and hospital LOS of 6 days (7). Park and colleagues published one of the largest series on robotic-assisted pulmonary lobectomy, with median operative time of 206 min, conversion-to-open rate of 8%, overall mobility rate of 25%, and median hospital LOS of 5 days (8).
How has BSA related to outcomes in other clinical scenarios? In transplant surgery, recipients with a higher (graft kidney volume):(recipient BSA) ratio had better graft function than those with lower ratios at 1 and 6 months post-transplantation (9). Both female gender and small BSA are associated with higher degrees of hemodilution during cardiac bypass, which may directly deteriorate outcomes and lead to transfusions as a result of low hemoglobin levels during and after the operation (10). Komoda et al., revealed that adult candidates for heart transplant with lower BSA, including most female patients, had worse prognosis on the waiting list after progression to critically ill status (11). Patients with smaller BSA have worse outcomes after various cardiovascular interventions due to smaller size and calibers of their vascularity (4). In contrast, completion of laparoscopic colorectal procedures may be more technically challenging and time consuming in large BSA patients (12).
Using BSA as surrogate for chest wall area and the operative field during thoracoscopic surgery, our study investigated the relationship between BSA and perioperative outcomes of robotic-assisted VATS lobectomy. Robotic instrumentation provides more degrees of freedom to the surgeon to perform difficult resections. In our experience, smaller operative fields resulted in problems with robotic arms colliding and making robotic-assisted VATS surgery more challenging, as confirmed by greater overall conversion-to-thoracotomy rates in patients with smaller BSA. However, even though smaller BSA represents a challenge in manipulation of robotic instrumentation, perioperative outcomes after robotic-assisted VATS lobectomy did not seem to be worsened by smaller BSA.
Conclusions
Patients with BSA≤1.65 m2 have a higher proportion of women and have lower BMI than patients with larger BSA. While patients with BSA ≤1.65 m2 have higher overall conversion rates than patients with larger BSA, emergent conversion rates for bleeding is similar between the two BSA groups. Moreover, smaller BSA patients have similar operative times, EBL, overall intraoperative complications, overall postoperative complications, chest tube days, hospital LOS, and in-hospital mortality rates as patients with larger BSA. Our study suggests that robotic-assisted pulmonary lobectomy is feasible and safe in patients with small body habitus.
Acknowledgements
None.
Footnote
Conflicts of Interest: Eric M. Toloza and Jacques-Pierre Fontaine had financial relationships with Intuitive Surgical Corporation in form of honoraria as robotic thoracic surgery proctors and observation sites. This paper is an update of previous Oral Presentations at the 51st Annual Meeting of the Eastern Cardiothoracic Surgical Society, Clearwater, FL, October 24, 2013, and at the 1st Annual World Robotic Symposium of the Society of Robotic Surgery, Lake Buena Vista, FL, November 9, 2013.
References
- Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987;317:1098. [Crossref] [PubMed]
- Verbraecken J, Van de Heyning P, De Backer W, et al. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism 2006;55:515-24. [Crossref] [PubMed]
- Stobäus N, Küpferling S, Lorenz ML, et al. Discrepancy between body surface area and body composition in cancer. Nutr Cancer 2013;65:1151-6. [Crossref] [PubMed]
- Messé SR, Kasner SE, Mehta Z, et al. Effect of body size on operative risk of carotid endarterectomy. J Neurol Neurosurg Psychiatry 2004;75:1759-61. [Crossref] [PubMed]
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-95. vi-vii. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [PubMed]
- Takagi H, Yamamoto H, Goto SN, et al. Perioperative results of robotic lung lobectomy: summary of the literature. Surg Endosc 2012;26:3697-9. [Crossref] [PubMed]
- Park BJ. Robotic lobectomy for non-small cell lung cancer (NSCLC): Multi-center registry study of long-term oncologic results. Ann Cardiothorac Surg 2012;1:24-6. [PubMed]
- Lee JH, Won JH, Oh CK. Impact of the ratio of graft kidney volume to recipient body surface area on graft function after live donor kidney transplantation. Clin Transplant 2011;25:E647-55. [Crossref] [PubMed]
- Ranucci M, Pazzaglia A, Bianchini C, et al. Body size, gender, and transfusions as determinants of outcome after coronary operations. Ann Thorac Surg 2008;85:481-6. [Crossref] [PubMed]
- Komoda T, Drews T, Hetzer R, et al. Adult candidates for heart transplantation with larger body surface area have better prognosis on waiting list after progression to critically ill status. Eur J Cardiothorac Surg 2011;39:317-22. [Crossref] [PubMed]
- Vaccaro CA, Vaccarezza H, Rossi GL, et al. Body surface area: a new predictor factor for conversion and prolonged operative time in laparoscopic colorectal surgery. Dis Colon Rectum 2012;55:1153-9. [Crossref] [PubMed]


