Icotinib combined whole brain radiotherapy for patients with brain metastasis from lung adenocarcinoma harboring epidermal growth factor receptor mutation
Introduction
Lung cancer is the leading cause of cancer deaths worldwide. Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases, and 15% to 30% of patients with NSCLC have reported developing brain metastasis (1,2). The incidence of brain metastasis has increased over the last decades as a result of the introduction of effective diagnostic methods and the promotion of public health awareness. However, the prognosis of NSCLC patients with brain metastasis remains poor, with the one-year overall survival (OS) rate being 10% only (3). Whole brain radiation therapy (WBRT) is considered the standard treatment for patients with brain metastasis. However, the outcome of WBRT for such patients remains poor, with a median OS time of three to six months (4,5). Satisfactory response rates and long survival times have been reported among patients who have received WBRT concomitant with temozolomide (6,7). The inability of traditional chemotherapy agents to cross the blood-brain barrier and their severe side effects hinder their application in the treatment of lung cancer patients with brain metastasis. However, wild-type patients with asymptomatic brain metastasis can be treated with upfront chemotherapy, the cerebral response rate of which is slightly lower than the extracranial response rate. A meta-analysis indicated that the concomitant use of WBRT and chemotherapy might increase the treatment response rate for brain metastasis with limited toxicity, but the result failed to reveal any significant improvement in neurological progression and OS times (8).
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib and erlotinib, have been proven useful in treating EGFR-mutated patients with brain metastasis. However, their use as radiosensitizers has never been clearly validated in a purely wild-type population. Welsh et al. (9) reported that erlotinib plus WBRT could significantly improve the OS time of patients. The median OS time of patients with the EGFR mutation is 19.1 months. The concomitant use of WBRT and gefitinib is well tolerated with a median OS time of 12.0 to 15.4 months (10,11). Some studies reported that the blood-brain barrier permeability of EGFR-TKIs could be increased in accordance with the escalated dose of WBRT (11,12). The cerebrospinal fluid-to-plasma ratio of NSCLC patients with brain metastasis reaches its peak (1.87%±0.72%) at a WBRT dose of 30 Gy (11). A phase II study (13) investigated the efficacy of EGFR-TKIs in treating brain metastasis among NSCLC patients with EGFR mutations. Of the 28 patients, 23 patients (83%) showed a partial response (PR) to the inhibitors. The median PFS and OS times were 6.6 and 15.9 months, respectively.
Icotinib is a new oral selective EGFR-TKI that has been approved for treating advanced NSCLC. Zhang et al. (14) recently investigated a patient with lung adenocarcinoma (LAC) and brain metastasis who received icotinib treatment. The EGFR mutation status showed a point mutation at exon 21 (L858R). The patient survived for one year and is still living. In our study, we retrospectively analyzed 43 patients with brain metastasis from LAC, as well as EGFR mutations. These patients were treated with icotinib and WBRT simultaneously. This study aims to assess the survival of these patients and identify the prognostic factors.
Methods
Patients
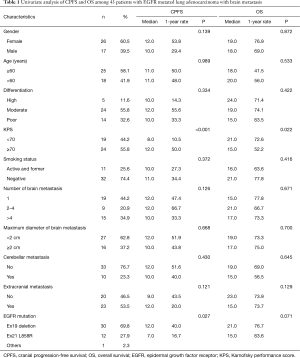
From February 2011 to December 2013, 43 LAC patients (Jiaxing First Hospital) with EGFR mutations that developed into brain metastasis were enrolled in this retrospective study. This study was approved by the Ethical Committee of the Jiaxing First Hospital. Informed consents were obtained from all patients or their next of kin (if the patient died). The eligibility criteria included histologically or cytologically confirmed LAC with the EGFR mutation, newly diagnosed brain metastasis based on contrast-enhanced MRI, suitability for brain metastasis treatment response evaluation, Karnofsky performance score (KPS) >60, no history of using EGFR-TKIs, aged above 18 years, no previous cranial radiotherapy, and normal hematologic, renal, and hepatic functions seven days before the protocol treatment. The age, sex, smoking status, KPS, tumor cell differentiation, and metastatic brain tumor characteristics were recorded for each patient by reviewing their medical charts and radiological images. Non-smokers were defined as those who had smoked less than 100 cigarettes in their lifetime. KPS was recorded on the first day of the WBRT. The brain metastasis characteristics, including the presence of cerebellar metastasis, extracranial metastasis, number of brain metastases, and maximum diameter of brain metastasis, were recorded a week before the WBRT. The patients were grouped by age (≥60 and <60 years), tumor cell differentiation (high, moderate, and poor), KPS (≥70 and <70), smoking status (active/formerly active and negative), number of brain metastases (1, 2 to 4, and >4), maximum diameter of brain metastasis (<2 and ≥2 cm), cerebellar metastasis (yes and no), extracranial metastasis (yes and no), and EGFR mutation (Ex19 deletion, Ex21 L858R, and others).
EGFR mutation examination
EGFR gene mutations were examined in paraffin-embedded surgical tissue sections from the primary LAC. The mutational analyses of the EGFR gene exons 18, 19, 20, and 21 were performed via polymerase chain reaction-based direct sequencing (15).
Treatment
After their enrollment in the study, all the patients received standard WBRT administered in 30 Gy to the whole brain in 10 daily fractions. The treatment was performed with a linear accelerator with an energy of 6 MV photons. Meanwhile, each patient took 125 mg icotinib thrice a day beginning from the first day of the WBRT. After completing the WBRT, maintenance icotinib (also at 125 mg thrice a day) was administered until the disease progressed or intolerable adverse effects were observed. To evaluate the clinical response of these patients to WBRT and icotinib, whole brain MRI and extracranial CT were performed four weeks following the completion of the WBRT and every three months thereafter or at the onset of neurological symptom deterioration. To evaluate their treatment response, those patients who underwent post-treatment brain MRI or CT were assessed according to the RECIST criteria (16). The toxicities were documented according to the Common Terminology Criteria for Adverse Events Version 3.0 once a week during treatment and then every three months during follow-up visits. The toxicities were recorded as the highest grade experienced during the whole treatment.
Statistical analysis
The primary endpoints of this study were cranial progression-free survival (CPFS) and OS. CPFS referred to the period starting from the first day of the WBRT to the progression of brain disease. OS referred to the period starting from the first day of the WBRT to death or censoring. The secondary endpoints included safety and treatment response. The Kaplan-Meier method was used to calculate survival rate while the log-rank test was performed to determine statistically significant differences. All statistical analyses were performed using SPSS 18.0 (Inc, Chicago, IL, USA). The P values of <0.05 indicate statistical significance.
Results
Characteristics of patients
Forty-three patients who developed brain metastasis between February 2011 and December 2013 and followed-up for at least six months were considered eligible for the brain metastasis treatment response evaluation. Table 1 summarizes the demographic and clinical characteristics of these patients. The median age at the time of brain metastasis was 62 years (range: 41 to 78 years), whereas the median follow-up duration was 16 months. EGFR mutations were determined in all 43 patients, including 30 cases of exon 19 mutation, 12 cases of exon 21 L858R mutation, and 1 case of exon 20 S768I mutation. At the last follow-up visit, 30 patients (69.8%) had died and 13 patients (30.2%) were still living.
Full table
Clinical response and toxicity
All patients underwent WBRT. During the first evaluation (four weeks after the completion of WBRT), 2 patients (4.7%) presented a complete response (CR), whereas 20 patients (46.5%) presented a PR. Nineteen patients showed stable disease (SD), whereas two patients showed progressive disease (PD). The disease control rate, calculated as CR + PR + SD, was 95.3%.
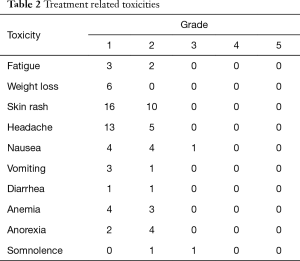
All 43 patients with brain metastasis could tolerate the combination of icotinib and WBRT. The most common type of toxicity was a grade 1–2 skin rash, which occurred among 26 patients (60.5%). A grade 1 or 2 headache was observed in 18 patients and could be relieved using mannitol. No grade 4 or 5 treatment-related toxicities were observed (Table 2).
Full table
Association between clinical variables and patient prognosis by univariable and multivariable analysis
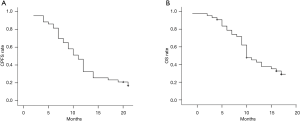
The median CPFS and OS times were 11.0 and 15.0 months, respectively. The six-month, one-year, and two-year CPFS rates were 81.4%, 32.6%, and 16.7%, respectively (Figure 1A), whereas the six-month, one-year, and two-year OS rates were 95.3%, 74.0%, and 29.0%, respectively (Figure 1B).
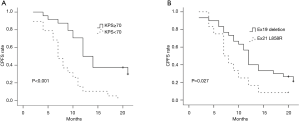
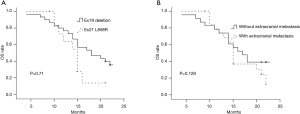
In the univariate analysis, improved CPFS was associated with KPS ≥70 (P<0.001) and EGFR exon19 deletion mutation (P=0.027). The median CPFS time was shorter among the patients with KPS <70 than among those with KPS ≥70 (12.0 vs. 7.0 months, P<0.001, Figure 2A). The one-year CPFS rate was 40.0% for the patients harboring EGFR exon19 deletion and 16.7% for the patients with EGFR exon 21 L858R (P=0.027, Figure 2B). The patients whose brain metastases showed the maximum diameter of <2 and ≥2 cm demonstrated similar CPFS times (12.0 vs. 10.0 months, P=0.668) as those patients with or without cerebellar metastasis (12.0 vs. 10.0 months, P=0.430) and extracranial metastasis (9.0 vs. 12.0 months, P=0.121). A borderline significant difference was observed in the OS of the patients harboring EGFR exon 19 deletion and those with EGFR exon 21 L858R (median OS time: 21.0 vs. 15.0 months, P=0.071, Figure 3A). Similarly, the patients with extracranial metastasis had a shorter OS time than those without extracranial metastasis. However, the P value was marginal (P=0.129, Figure 3B). Table 1 summarizes the univariate analysis results.
Discussion
The brain is a metastatic organ that is prone to LAC. About 25% of patients develop brain metastasis within two years after their diagnosis. However, the outcome of patients with brain metastasis remains very poor, and these patients only have a median survival time of four weeks to seven weeks without treatment (17). The efficacy of most potential agents for brain tumors is often limited by the presence of the blood-brain barrier. WBRT with a 20 to 30 Gy dosage may increase the permeability of this barrier (18) and prolong the median OS time to three months to five months (19,20). Concurrent WBRT and cisplatin-based chemotherapy can prolong the median OS time to 9.7 months (21). The LAC patients with brain metastasis who received a combination of pemetrexed and cisplatin/carboplatin reported a median OS of 11.0 months (22). The overexpression of EGFR is related to radiation resistance. The blockade of EGFR can reduce radiation resistance by reducing DNA repair, proliferation, and antiapoptosis (23). EGFR-TKIs, which are small molecular inhibitors of the EGFR pathway, have been proven much more effective than cisplatin-based chemotherapy in the progression-free survival of NSCLC patients (24). WBRT can also increase the blood-brain barrier permeability of gefitinib (11). Therefore, EGFR-TKIs are effective in the management of NSCLC patients with brain metastasis. Several reports recently demonstrated that NSCLC patients with brain metastasis can benefit from EGFR-TKI concurrent with or without WBRT. Park et al. (13) found that 23 of the 28 patients with NSCLC brain metastasis who were treated with erlotinib or gefitinib showed a PR. The median PFS and OS times of these patients were 6.6 and 15.9 months, respectively. No differences in survival were observed according to the used agents. Iuchi et al. (25) reported a treatment response rate of 87.8% when gefitinib without radiation therapy was used for patients with brain metastasis from EGFR-mutant LAC. The median PFS and OS times of these patients were 14.5 and 21.9 months, respectively. No patient experienced grade ≥4 toxicity. In another study (9), the concomitant use of WBRT and erlotinib for NSCLC brain metastasis was well tolerated by patients, with a median OS time of 11.8 months. Icotinib is a new type of highly selective EGFR-TKI that was approved by the Chinese Food and Drug Administration in 2011. A randomized double-blind phase 3 clinical trial demonstrated that icotinib and gefitinib had similar median PFS times of 4.6 and 3.4 months, respectively (P=0.13) (26). In our study, 43 LAC patients with brain metastasis and EGFR mutation were treated with WBRT plus icotinib. The median CPFS and OS times of these patients were 11.0 and 15.0 months, respectively. In a prospective study, Welsh et al. (9) found that the concomitant use of WBRT and erlotinib for NSCLC brain metastasis was well tolerated by the patients, with an overall treatment response rate of 86% and a median OS time of 11.8 months. Additionally, among the 17 patients with EGFR status, those with the EGFR mutations demonstrated a median OS time of 19.1 months, whereas those with wild-type EGFR mutations had a median OS time of 9.3 months. Similarly, in the retrospective study of Hsiao et al. (27), 139 LAC patients, including 89 EGFR mutant and 50 EGFR wild-type patients, were treated with EGFR-TKIs, and around 85% of these patients received radiotherapy. Among the EGFR-mutant patients, those who were treated with WBRT and EGFR-TKIs showed a higher treatment response rate than those who were only treated with EGFR-TKIs. The authors hypothesized that EGFR could regulate DNA repair dynamics and that NSCLC-harboring patients with the EGFR mutation might be more sensitive to radiotherapy than those with wild-type EGFR. Consistent with our study, the retrospective study of Gong et al. (28) showed that those patients with leptomeningeal metastasis from EGFR-mutated NSCLC who were treated with icotinib had a median OS time of 10.1 months.
Those patients with exon 19 deletion demonstrated a longer CPFS than those who harbored exon 21 L858R after the treatment with icotinib plus WBRT. The median CPFS time of those patients with exon 19 deletion was substantially increased to 12.0 months. In a meta-analysis (29), those patients with exon 19 deletion showed a longer PFS than those with exon 21 L858R mutations (HR =0.75, 95% CI, 0.65 to 0.85; P<0.001). Li et al. (30) reviewed 106 NSCLC patients with brain metastasis from NSCLC. The median OS time was shorter among the patients with the exon 21 point mutation than among those with the exon 19 deletion (10.4 vs. 15.0 months). However, only 42 patients (39.6%) in this study received the EGFR-TKI treatment. Although the roles of the exon 19 deletion and exon 21 L858R mutation in EGFR mutation remain controversial, our results, which are in line with those of previous studies (29,30), show that NSCLC with exon 19 deletions or NSCLC with exon 21 L858R are two types of malignancies with different clinical characteristics and pathogenesis. The exact mechanisms for achieving satisfactory outcomes in exon 19 deletion remain unclear. These outcomes may be attributed to the fact that the crystal structure of exon 21 L858R is more stable than that of the exon 19 deletion (31). Moreover, exon 21 L858R may be significantly related to the EGFR T790M mutation, which is associated with the acquired resistance to EGFR-TKIs (32).
This study has several limitations. First, our study included a comparative homogeneous population whose treatment response can be evaluated, thereby causing selection bias. Second, this retrospective study only included patients from one center, thereby allowing selection bias. A prospective study must be performed to validate our results and to confirm the efficacy of icotinib in controlling brain metastases.
In summary, this clinical study shows that the concurrent administration of icotinib and WBRT for treating brain metastasis from EGFR-mutated LAC is safe and tolerable. After the treatment, the patients with the EGFR exon 19 deletion demonstrated a longer CPFS than those with the exon 21 L858R mutation. Large-scale multi-center randomized trials must be performed to validate these results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Ethical Committee of The First Hospital of Jiaxing and written informed consent was obtained from all patients.
References
- Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002;94:2698-705. [Crossref] [PubMed]
- Smedby KE, Brandt L, Bäcklund ML, et al. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer 2009;101:1919-24. [Crossref] [PubMed]
- Hsiung CY, Leung SW, Wang CJ, et al. The prognostic factors of lung cancer patients with brain metastases treated with radiotherapy. J Neurooncol 1998;36:71-7. [Crossref] [PubMed]
- Kepka L, Cieslak E, Bujko K, et al. Results of the whole-brain radiotherapy for patients with brain metastases from lung cancer: the RTOG RPA intra-classes analysis. Acta Oncol 2005;44:389-98. [Crossref] [PubMed]
- Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol 2005;23:6207-19. [Crossref] [PubMed]
- Antonadou D, Paraskevaidis M, Sarris G, et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol 2002;20:3644-50. [Crossref] [PubMed]
- Verger E, Gil M, Yaya R, et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Radiat Oncol Biol Phys 2005;61:185-91. [Crossref] [PubMed]
- Qin H, Pan F, Li J, et al. Whole brain radiotherapy plus concurrent chemotherapy in non-small cell lung cancer patients with brain metastases: a meta-analysis. PLoS One 2014;9:e111475. [Crossref] [PubMed]
- Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013;31:895-902. [Crossref] [PubMed]
- Lin CH, Hsu KH, Chang SN, et al. Increased survival with the combination of stereotactic radiosurgery and gefitinib for non-small cell lung cancer brain metastasis patients: a nationwide study in Taiwan. Radiat Oncol 2015;10:127. [Crossref] [PubMed]
- Zeng YD, Liao H, Qin T, et al. Blood-brain barrier permeability of gefitinib in patients with brain metastases from non-small-cell lung cancer before and during whole brain radiation therapy. Oncotarget 2015;6:8366-76. [Crossref] [PubMed]
- Deng Y, Feng W, Wu J, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol 2014;2:116-120. [PubMed]
- Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012;77:556-60. [Crossref] [PubMed]
- Zhang Y, Tang H, Li J, et al. An active treatment of lung adenocarcinoma cancer with brain metastases: icotinib. Onco Targets Ther 2015;8:1351-4. [Crossref] [PubMed]
- Chiu CH, Ho HL, Chiang CL, et al. Clinical characteristics and treatment outcomes of lung adenocarcinomas with discrepant EGFR mutation testing results derived from PCR-direct sequencing and real-time PCR-based assays. J Thorac Oncol 2014;9:91-6. [Crossref] [PubMed]
- Lei YY, Yang JJ, Zhong WZ, et al. Clinical efficacy of crizotinib in Chinese patients with ALK-positive non-small-cell lung cancer with brain metastases. J Thorac Dis 2015;7:1181-8. [PubMed]
- Chi A, Komaki R. Treatment of brain metastasis from lung cancer. Cancers (Basel) 2010;2:2100-37. [Crossref] [PubMed]
- van Vulpen M, Kal HB, Taphoorn MJ, et al. Changes in blood-brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy? Oncol Rep 2002;9:683-8. (Review). [PubMed]
- Khuntia D, Brown P, Li J, et al. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol 2006;24:1295-304. [Crossref] [PubMed]
- Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 1999;43:795-803. [Crossref] [PubMed]
- Furuse K, Kamimori T, Kawahara M, et al. A pilot study of concurrent whole-brain radiotherapy and chemotherapy combined with cisplatin, vindesine and mitomycin in non-small-cell lung cancer with brain metastasis. Br J Cancer 1997;75:614-8. [Crossref] [PubMed]
- Zhu W, Røe OD, Wu C, et al. Activity of pemetrexed-based regimen as first-line chemotherapy for advanced non-small cell lung cancer with asymptomatic inoperable brain metastasis: a retrospective study. J Chemother 2015;27:221-6. [Crossref] [PubMed]
- Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res 2005;65:3328-35. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 2013;14:953-61. [Crossref] [PubMed]
- Hsiao SH, Lin HC, Chou YT, et al. Impact of epidermal growth factor receptor mutations on intracranial treatment response and survival after brain metastases in lung adenocarcinoma patients. Lung Cancer 2013;81:455-61. [Crossref] [PubMed]
- Gong L, Xiong M, Huang Z, et al. Icotinib might be effective for the treatment of leptomeningeal carcinomatosis in non-small cell lung cancer with sensitive EGFR mutations. Lung Cancer 2015;89:268-73. [Crossref] [PubMed]
- Zhang Y, Sheng J, Kang S, et al. Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS One 2014;9:e107161. [Crossref] [PubMed]
- Li H, Zhang X, Cao J, et al. Erratum to: Exon 19 deletion of epidermal growth factor receptor is associated with prolonged survival in brain metastases from non-small-cell lung cancer. Tumour Biol 2015;36:7333-4. [Crossref] [PubMed]
- Shan Y, Eastwood MP, Zhang X, et al. Oncogenic mutations counteract intrinsic disorder in the EGFR kinase and promote receptor dimerization. Cell 2012;149:860-70. [Crossref] [PubMed]
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. [Crossref] [PubMed]


