Randomized trial of epidural vs. subcutaneous catheters for managing pain after modified Nuss in adults
Introduction
Pectus excavatum (PE) is the most common chest wall deformity and repair is recommended for severe symptoms (1,2). Minimally invasive repair of pectus excavatum (MIRPE), or modified Nuss, has become standard of care for children and its use has recently been extended to adult patients (3-6). Increased chest wall rigidity in adults may be associated with higher complication rates, technical difficulties and increased postoperative pain (4,7-10). Hospitalization may be prolonged in adult patients after MIRPE due to uncontrolled pain (8,11,12). Thoracic epidural analgesia (TEA) and intravenous patient-controlled analgesia (PCA) are effective in younger patients; however, reports of postoperative pain regimens in adults are limited (11,13-17). Tunneled, anesthetic-infiltrating catheters are being used increasingly after thoracic procedures such as thoracotomy in lieu of TEA with some reports showing earlier hospital discharge for patients who receive this type of analgesia (18-20). In the past, TEA was used routinely in our adult MIRPE patients. In an effort to improve outcomes and decrease length of stay (LOS), bilateral tunneled catheters infusing local anesthetic were initiated. This prospective, randomized trial was performed to determine if bilateral subcutaneous local anesthetic catheters would provide adequate analgesia with improved outcomes versus TEA.
Methods
Patients
Institutional Review Board approval and patient informed consent obtained before enrollment. In total, 163 adult patients (age ≥18 years) underwent PE repair from August 2013–April 2015, with 85 patients enrolled for randomization. Exclusion criteria included <18 years, previous PE repair with extensive recurrence, history of chronic pain requiring opioids or refusal of randomization. Patients deemed inappropriate for safe placement of TEA catheter were also excluded.
Study design
This was a prospective, randomized, single-center trial of patients undergoing MIRPE for PE. Patients were enrolled using computer-generated randomization to either: continuous infusion of local anesthetic at surgical wound site through On-Q pump with a Select-A-Flow Variable Rate Controller (On-Q Pain Relief System, Halyard Health, Inc., Irvine, CA, USA) or TEA with local anesthesia. Patients also received standardized perioperative analgesic medication protocol which is highlighted in the Box 1. An individual unit of randomization was used in a non-stratified sequence in blocks of four. After consent for study enrollment, the randomization sequence was accessed to identify next allocation group.

Full table
Primary outcomes were postoperative pain score and opioid use and length of hospitalization. Patients rated their postsurgical pain on a visual analog scale [0 (no pain) to 10 (worst pain)] every 15 minutes in the recovery room, at least every 4 hours during hospitalization, and then in a journal for 7 days after discharge. Demographic data, medications, and surgical/medical history were obtained by patient interview and electronic medical record.
Hospitalization data was also obtained from electronic medical records; post discharge data (pain, opioid use, complications) was obtained from patient self-report journals and postoperative follow-up appointments. Opioid medication was converted to morphine-equivalent units (mg), using an equianalgesic opioid dose table (21).
The power was based on a 2-sample t-test for group means comparison between the thoracic epidural group and the On-Q group. Assuming the thoracic epidural group had a mean LOS of 5.5 days and a standard deviation of 3.0 days, a sample of 50 patients per group provided 80% power to detect a 1.7 day (or 0.57 standard deviation), and also to detect a 0.6 standard deviation difference in AUC between the thoracic epidural group and the On-Q group.
Statistical analyses
Descriptive summaries included frequencies and proportions for categorical variables, and mean, standard deviation and range for continuous variables. The two groups were compared by using the independent 2-sample t-test (unequal variance) for continuous variables and Fisher’s exact test for categorical variables. A mixed-effects model (including only days 1–7) with each subject as random effect was used to model postoperative pain and opioid use to account for the correlation due to multiple observations (days) for each subject. The fixed effects in the model were group, day, and the 2-way interaction (day × group). The estimated least-squares means (adjusted means) estimated from the model and their corresponding 95% confidence intervals were also computed for pain scores and morphine use. Pain scores are expressed as the mean of 12-hour segment. Significance was defined at 0.05 level. Statistical analyses were performed using the statistical software packages SAS Studio version 9.3 (SAS Institute, Cary, NC, USA), and R version 3.1.2.
Analgesic protocol
Perioperative analgesic medications were standardized for both groups (Box 1). Patients in both arms of study received infusion of local anesthetic only through TEA or On-Q catheters. Both groups also received the protocol’s standardized pain regimen (Figure 1).
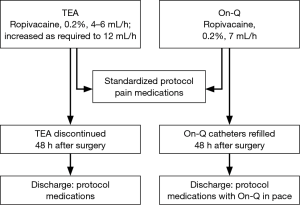
Patients randomized to TEA had thoracic epidural catheter placed by 1 of 5 attending cardiothoracic anesthesiologists via midline or paramedian approach at T5-T6/T6-T7 using standard loss-of-resistance technique. Catheter was tested with 3-mL 1.5% lidocaine with epinephrine (1:200,000) to exclude intravascular or intrathecal placement. Epidural infusion of 0.2% ropivacaine was started at 4–6 mL/hour during surgical procedure, and was titrated via a standard order set by the hospital nursing staff. The order set included a range from 6–12 mL/hour with instructions to increase continuous infusion by 1 mL each hour for moderate to severe pain. Epidural catheters were left in place for 48 hours and removed on postoperative day 2 to assist in ambulation and transition to oral opioids and discharge unless the patient had inadequate pain control with oral medications or there were complications with the epidural catheter requiring early removal.
Patients randomized to On-Q had multi-holed, 7.5-cm wound catheters inserted bilaterally by the thoracic surgeon at end of surgical procedure. Catheters were inserted subcutaneously with the help of tunnelers and advanced in a plane just superficial to the rib and lateral to the axilla and surgical site as described for analgesia use post sternotomy and rib fracture (22,23). Each catheter infused 0.2% ropivacaine and locked at rate of 7 mL/hour. The On-Q catheters were primed and attached to 750-mL, fill-volume reservoir, which was refilled after 48 hours (when it was nearly empty). The catheters were left in place for 7 days maximum. Patients were discharged home with On-Q pump system unless they asked to have it removed.
When it occurred, pruritus was managed with 50 mg diphenhydramine. Severe muscle spasm was treated first with 5 mg cyclobenzaprine, and then 2.5–5.0 mg diazepam if needed for rescue. Nausea or vomiting was treated with ondansetron and, if needed, a rescue antiemetic (prochlorperazine or promethazine).
Discharge criteria required adequate analgesia (pain score, ≤4) with oral medication protocol (Box 1) for 24 hours without substantial cognitive or respiratory adverse effects. If intravenous opiates or increasing doses of extended release morphine were needed to supplement standard medications, discharge was delayed for another 24 hours. Discharge was also delayed for postoperative complications, such as ileus, nausea and vomiting, pneumonia, or other medical issues. Patients were discharged home with standardized analgesic regimen described in the Box 1.
Surgical procedure
We used modified Nuss procedure (MIRPE) in all patients, which has been previously described (24,25) and explained briefly below. All patients received prophylactic intravenous antibiotic before procedure. General anesthesia was induced by using single dose of intravenous fentanyl, 0.5–2.0 mcg/kg; lidocaine, 1–2 mg/kg; and propofol, 2–4 mg/kg. Endotracheal intubation with double-lumen tube was facilitated with rocuronium, 0.6–1.2 mcg/kg. General anesthesia was maintained by sevoflurane. A 1-time dose of intravenous methadone, 0.20–0.35 mg/kg, was administered at beginning of surgical procedure.
Patients were positioned supine with back gel rolls parallel to spine and arms secured at sides. ChloraPrep (CareFusion Corp, San Diego, CA, USA) was used after which Ioban (3M, St. Paul, MN, USA) antimicrobial incise drapes were applied. Bilateral incisions were made at inferolateral pectoral border and subpectoral pockets fashioned. Ports (5 mm) were placed through right incision and an inferior-lateral incision above the diaphragm, for thoracoscope 2-mm incisions were placed on either side of the sternal defect. Perforating tips of bone clamp [Lewin Spinal Perforating Forceps (V. Mueller], CareFusion, Inc.] were inserted into sternum, and cable attached to Rultract table-mounted retractor (Rultract Inc., Cleveland, OH, USA) (24). Sternum was then elevated.
Next, dissection was done across mediastinum and into left side of thoracic cavity. The Lorenz dissector (Lorenz Surgical Inc., Jacksonville, FL, USA) was passed into an interspace and guided through corresponding interspace on the left. A #5 FiberWire (Arthrex, Inc., Naples, FL, USA) was attached to the passer as it was withdrawn and used to guide support bar into position. Each bar was circumferentially fixed around ribs at multiple sites bilaterally with FiberWire. Sternum was released and clamp removed. Pectoralis muscles reapproximated over bars and incisions closed with absorbable suture.
The surgeon then placed On-Q catheters by using disposable 17-gauge × 8-inch tunneling system (Model T17X8, Halyard Health). The 7.5-cm soaker catheters (MP050-A, Halyard Health) were placed bilaterally anterior to the ribs and tunneled lateral to surgical site along the axilla (Figure 2). All patients also had bilateral intercostal block [bupivacaine hydrochloride, 0.25% (1 mL/kg)] and received intravenous ketorolac, 30 mg, and ondansetron, 4 mg at procedure completion.
Results
A total of 85 patients were randomized. Of patients randomized to epidural arm, 13 withdrew their consent (refused epidural placement on day of surgery), and two patients were excluded because we could not place epidural catheter satisfactorily. Of patients randomized to On-Q arm, two were excluded (one declined to participate on day of surgery; we learned after randomization that the other had history of chronic use of pain medications). After analyzing data, we did not find any significant differences between TEA and On-Q groups for patients who withdrew from the study; however, those who did not withdraw had longer mean hospitalization (P=0.02). Patient enrollment and demographic data of all 68 patients completing the study are summarized in Table 1. There were no differences between groups. In particular, both groups had similar lengths of stay (P=0.55). Mean hospital charges were not significantly different between the two cohorts (P=0.10).
Full table
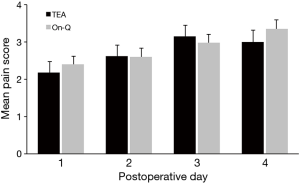
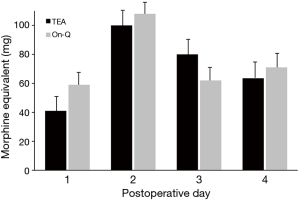
The epidural catheter was removed on postoperative day 2 in 74% of patients (20/27). Three patients had epidural removed day 1 due to leakage around catheter insertion site. Three patients had epidural removed day 3 and one patient day 4 due to pain that was not adequately controlled and early removal was not felt to be in the patient’s best interest. The On-Q pump remained in place for up to 7 days, including after patients were discharged. We compared pain and morphine use between the two groups through postoperative day 4; however, only postoperative days 1 and 2 were directly comparable due to epidural removal. Most patients were discharged home by day 4. Home-journal entries for pain and morphine use were not sufficient for analysis; only 27 (40%) of patients completed the outpatient assessment for additional 7 days’ evaluation of opioid use and pain scores. Overall, there was no difference between study groups in mean pain scores (Figure 3) or morphine-equivalent use (Figure 4). However, there was significant increase overall in daily mean pain scores for both groups (day effect, P<0.001).
Complications
Complications were not significantly different between groups (Table 2). Two patients, one from each group were treated with antibiotic for unconfirmed, but suspected pneumonia, due to elevated white count and atelectasis versus infiltrate on imaging. Two patients from the On-Q group also were diagnosed with urinary tract infections. No other infections occurred during patients’ hospitalization or within 30 days of their surgery. Patients in both groups had urinary retention.

Full table
Discussion
Postoperative pain after MIRPE, which has been shown to be a challenge in young patients (11,26), could be an even greater issue in adults who have less chest wall flexibility. Pain may cause prolonged hospitalization of adult patient with some centers reporting up to mean of 10 days in their older patient cohorts (8). TEA has been standard method for pain management in adults undergoing thoracic procedures, but it is associated with risk of neurologic complications in up to 0.07% of patients (15,26). Pain may also increase after epidural catheter removal, which may delay hospital discharge while oral medications are adjusted to appropriate levels (11,18,27). In our early adult MIRPE experience, achieving adequate analgesia was difficult and the use of PCA over TEA was not found to be an advantage as has been reported for children recovering after MIRPE (14). PCA on-demand dosing alone was unsuccessful at maintaining acceptable pain levels and a number of patients experienced complications related to respiratory depression and severe sedation when continuous background dosing was used. We had also experienced issues with significant hypotension and respiratory suppression utilizing TEA with both opioid and local anesthetic at infusion levels high enough to provide adequate analgesia.
In an effort to reduce complications, including opioid-induced respiratory depression, and decrease hospital LOS, we began utilizing TEA with local anesthetic and on-demand PCA. Others have reported successful use of epidural infusion of local anesthetic with concomitant intravenous PCA (28). Although TEA with PCA was successful, the use of On-Q catheters with PCA was another attractive option which has been reported by others for rib fractures and postoperative sternotomy analgesia utilizing subcutaneous insertion (22,23). This study was designed to compare postoperative pain management and outcomes between TEA and bilateral subcutaneous infusion pump catheters (On-Q) as an adjunct to the on demand PCA. Transition to oral opioids was planned the first postoperative day with discharge anticipated by day 3. The use of longer-acting opioids intraoperatively, such as methadone, and nonopioid multimodal analgesia, may also contribute to decrease in postoperative pain.
We found that both pain and morphine-equivalent opioid use were similar between groups (P=0.52; P=0.28, respectively). A pattern of increasing mean pain scores occurred over postoperative days, but there was no group or interaction effect (P=0.66). This daily increase in pain scores may be secondary to patients’ increased activity. Only 27 of 68 patients (40%) returned adequately maintained pain-and-opioid-use journals after they were discharge from hospital, which limited our ability to assess data up to 7 days after discharge.
Another objective of our study was to determine whether a specific analgesic regimen was associated with shorter hospitalization time. Hospital LOS varies greatly among institutions where PE is done, with mean durations of 4 to 10 days common globally (11,17,29-32). Our two treatment groups had a short mean LOS that was without significant difference [On-Q, 3.3 days vs. TEA, 3.5 days (P=0.55)]. By protocol, patients were required to have 24 hours of documented, adequate pain score (≤4) on oral medications before they could be discharged. Pain and opiate use did not significantly increase in TEA group after catheter removal as has been reported by others (17,33) however in four patients, attempts to wean epidural dosing for removal resulted in inadequate analgesia with standard oral pain medication dosing and the epidural was left in for additional 24–48 hours. The optimal time to discontinue TEA is unknown. Others have reported 3 to 5 days use of epidural analgesia, which substantially increases hospital stay (11,17,19,34). In our cohort, all patients were planned for discharge by day 3. Therefore, removal of TEA catheter was scheduled for postoperative day 2 although some patients required longer analgesia.
Although we enrolled the same number of patients in each group at randomization, the On-Q arm was larger because 13 patients (15.3%) in TEA group voluntarily withdrew before treatment due to perceived risks of epidural catheter placement and perceived benefits of On-Q pain management system (14,18,20). Despite this imbalance, the groups were not significantly different in regard to characteristics compared. We believe social networking and information availability on the Internet negatively affected our ability to enroll and randomize patients. Patients in both groups had preexisting, strong opinions about pain control, and only 52% (85/165) of patients undergoing surgery for PE were willing to undergo randomization or met the eligibility criteria for study inclusion. This issue with enrollment is a critical lesson to be learned from this study, and it reinforces the difficulty of conducting randomized trials in surgery.
There are a number of weaknesses inherent in this study. The researchers were not blinded to the therapies, which could raise the potential for bias. A significant drop out occurred in the TEA group. We attempted to address this point by providing a group comparison between dropouts versus not. We did not find any significant differences between patients who withdrew from the study; however, those who did not withdraw had longer mean hospitalization (P=0.02).
A small number of patients (40%) completed the outpatient assessment after discharge; therefore, we were unable to draw any conclusions on pain after discharge. A patient satisfaction component was not included. Our intention was to compare the two methods we currently use to assess if one was superior. Our primary goal is to provide safe, adequate analgesia for our adult patients with PE who undergo MIRPE and to discharge them home as soon as possible. Both treatment arms had few complications and provided adequate pain relief with short hospital LOS. Our results suggest that in the presence of a comprehensive multimodal analgesic approach both On-Q and TEA produce similar results regarding patients’ pain, opioid use, and LOS. We did not observe a difference in complication rates, including infection, although the small size of this study limits our ability to detect minor differences.
Conclusions
In summary, postoperative pain management in adults after MIRPE can be challenging. We have had success with a balanced approach including a combination of opioid medications, nonopioid adjuncts, and local anesthetic delivery systems. In recommending any pain management system or treatment, however, we need to remember that our patients are educating themselves about the options, and their biases may influence decisions.
Acknowledgements
This work was supported in part by Halyard Health formerly Kimberly-Clark Health Care.
Footnote
Conflicts of Interest: Dawn E. Jaroszewski discloses consulting relationship with Zimmer Biomet. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Institutional Review Board of No. 12-008050 and patient informed consent obtained before enrollment.
References
- Obermeyer RJ, Goretsky MJ. Chest wall deformities in pediatric surgery. Surg Clin North Am 2012;92:669-84. ix. [Crossref] [PubMed]
- Jaroszewski D, Notrica D, McMahon L, et al. Current management of pectus excavatum: a review and update of therapy and treatment recommendations. J Am Board Fam Med 2010;23:230-9. [Crossref] [PubMed]
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. [Crossref] [PubMed]
- Croitoru DP, Kelly RE Jr, Goretsky MJ, et al. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Surg 2002;37:437-45. [Crossref] [PubMed]
- Pilegaard HK. Extending the use of Nuss procedure in patients older than 30 years. Eur J Cardiothorac Surg 2011;40:334-7. [PubMed]
- Kelly RE, Goretsky MJ, Obermeyer R, et al. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann Surg 2010;252:1072-81. [Crossref] [PubMed]
- Dzielicki J, Korlacki W, Janicka I, et al. Difficulties and limitations in minimally invasive repair of pectus excavatum--6 years experiences with Nuss technique. Eur J Cardiothorac Surg 2006;30:801-4. [Crossref] [PubMed]
- Kim DH, Hwang JJ, Lee MK, et al. Analysis of the Nuss procedure for pectus excavatum in different age groups. Ann Thorac Surg 2005;80:1073-7. [Crossref] [PubMed]
- Fonkalsrud EW, Reemtsen B. Force required to elevate the sternum of pectus excavatum patients. J Am Coll Surg 2002;195:575-7. [Crossref] [PubMed]
- Weber PG, Huemmer HP, Reingruber B. Forces to be overcome in correction of pectus excavatum. J Thorac Cardiovasc Surg 2006;132:1369-73. [Crossref] [PubMed]
- Papic JC, Finnell SM, Howenstein AM, et al. Postoperative opioid analgesic use after Nuss versus Ravitch pectus excavatum repair. J Pediatr Surg 2014;49:919-23; discussion 923. [Crossref] [PubMed]
- Molik KA, Engum SA, Rescorla FJ, et al. Pectus excavatum repair: experience with standard and minimal invasive techniques. J Pediatr Surg 2001;36:324-8. [Crossref] [PubMed]
- Gasior AC, Weesner KA, Knott EM, et al. Long-term patient perception of pain control experience after participating in a trial between patient-controlled analgesia and epidural after pectus excavatum repair with bar placement. J Surg Res 2013;185:12-4. [Crossref] [PubMed]
- St Peter SD, Weesner KA, Weissend EE, et al. Epidural vs patient-controlled analgesia for postoperative pain after pectus excavatum repair: a prospective, randomized trial. J Pediatr Surg 2012;47:148-53. [Crossref] [PubMed]
- Weber T, Mätzl J, Rokitansky A, et al. Superior postoperative pain relief with thoracic epidural analgesia versus intravenous patient-controlled analgesia after minimally invasive pectus excavatum repair. J Thorac Cardiovasc Surg 2007;134:865-70. [Crossref] [PubMed]
- McBride WJ, Dicker R, Abajian JC, et al. Continuous thoracic epidural infusions for postoperative analgesia after pectus deformity repair. J Pediatr Surg 1996;31:105-7; discussion 107-8. [Crossref] [PubMed]
- Ghionzoli M, Brandigi E, Messineo A, et al. Pain and anxiety management in minimally invasive repair of pectus excavatum. Korean J Pain 2012;25:267-71. [Crossref] [PubMed]
- Gebhardt R, Mehran RJ, Soliz J, et al. Epidural versus ON-Q local anesthetic-infiltrating catheter for post-thoracotomy pain control. J Cardiothorac Vasc Anesth 2013;27:423-6. [Crossref] [PubMed]
- Furrer M, Rechsteiner R, Eigenmann V, et al. Thoracotomy and thoracoscopy: postoperative pulmonary function, pain and chest wall complaints. Eur J Cardiothorac Surg 1997;12:82-7. [Crossref] [PubMed]
- Ried M, Schilling C, Potzger T, et al. Prospective, comparative study of the On-Q® PainBuster® postoperative pain relief system and thoracic epidural analgesia after thoracic surgery. J Cardiothorac Vasc Anesth 2014;28:973-8. [Crossref] [PubMed]
- PL Detail-Document, Opioid Conversion Algorithm. Pharmacist’s Letter/Prescriber’s Letter 2012. Available online: http://prescribersletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=MAYO&s=PRL&pt=2&fpt=2&dd=280821#PLVOICES4637
- Truitt MS, Murry J, Amos J, et al. Continuous intercostal nerve blockade for rib fractures: ready for primetime? J Trauma 2011;71:1548-52; discussion 1552. [Crossref] [PubMed]
- Eljezi V, Dualé C, Azarnoush K, et al. The analgesic effects of a bilateral sternal infusion of ropivacaine after cardiac surgery. Reg Anesth Pain Med 2012;37:166-74. [Crossref] [PubMed]
- Jaroszewski DE, Johnson K, McMahon L, et al. Sternal elevation before passing bars: a technique for improving visualization and facilitating minimally invasive pectus excavatum repair in adult patients. J Thorac Cardiovasc Surg 2014;147:1093-5. [Crossref] [PubMed]
- Jaroszewski DE, Ewais MM, Chao CJ, et al. Success of Minimally Invasive Pectus Excavatum Procedures (Modified Nuss) in Adult Patients (≥30 Years). Ann Thorac Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Giebler RM, Scherer RU, Peters J. Incidence of neurologic complications related to thoracic epidural catheterization. Anesthesiology 1997;86:55-63. [Crossref] [PubMed]
- Cucchiaro G, Adzick SN, Rose JB, et al. A comparison of epidural bupivacaine-fentanyl and bupivacaine-clonidine in children undergoing the Nuss procedure. Anesth Analg 2006;103:322-7. table of contents. [Crossref] [PubMed]
- Ong CC, Choo K, Morreau P, et al. The learning curve in learning the curve: a review of Nuss procedure in teenagers. ANZ J Surg 2005;75:421-4. [Crossref] [PubMed]
- Schalamon J, Pokall S, Windhaber J, et al. Minimally invasive correction of pectus excavatum in adult patients. J Thorac Cardiovasc Surg 2006;132:524-9. [Crossref] [PubMed]
- Aronson DC, Bosgraaf RP, van der Horst C, et al. Nuss procedure: pediatric surgical solution for adults with pectus excavatum. World J Surg 2007;31:26-9; discussion 30. [Crossref] [PubMed]
- Cheng YL, Lee SC, Huang TW, et al. Efficacy and safety of modified bilateral thoracoscopy-assisted Nuss procedure in adult patients with pectus excavatum. Eur J Cardiothorac Surg 2008;34:1057-61. [Crossref] [PubMed]
- Futagawa K, Suwa I, Okuda T, et al. Anesthetic management for the minimally invasive Nuss procedure in 21 patients with pectus excavatum. J Anesth 2006;20:48-50. [Crossref] [PubMed]
- Densmore JC, Peterson DB, Stahovic LL, et al. Initial surgical and pain management outcomes after Nuss procedure. J Pediatr Surg 2010;45:1767-71. [Crossref] [PubMed]
- Scheit MW, Litz RJ, Gaebler R, et al. Postoperative thoracic epidural analgesia in young adolescents undergoing Nuss' procedure for pectus excavatum repair. Anesthesiology 2005;103:A1387.


