Initial experience of single-port video-assisted thoracoscopic surgery sleeve lobectomy and systematic mediastinal lymphadenectomy for non-small-cell lung cancer
Introduction
Sleeve lobectomy (SL) and pneumonectomy are surgical options for the treatment of central-type non-small-cell lung cancer (NSCLC). Some studies reveal that, compared with pneumonectomy, SL is a more valid therapeutic option and offers better long-term survival and quality of life without increasing the morbidity and mortality in selected patients (1-3). However, the complexity of the procedure makes SL a technically challenging operation, and it was once considered a contraindication for video-assisted thoracoscopic surgery (VATS) in many institutions (4,5). In the past few years, some surgeons have become capable of performing SL via VATS because of their accumulated experience (6,7). Moreover, many case series have proved the safety and feasibility of VATS SL (6,8). Conventional VATS has been mostly performed with three or four incisions (8,9). The first case report of single-port VATS SL was by Gonzalez-Rivas et al. in 2013 (10), which proved it is possible to perform SL with fewer incisions. However, owing to the extensive alterations in operating technique required, single-port VATS SL is a more technically demanding operation, so most published data have been in the form of case reports (10,11).
After sufficient experience gained with conventional multi-port VATS, we have applied single-port VATS for the radical resection of NSCLC since May 2014 (12). To date, more than 400 single-port VATS, including wedge dissection, lobectomy, segmentectomy, SL, and other complex pulmonary resections, have been performed in our institution. Here, we present our current experience with single-port VATS SL and systematic mediastinal lymphadenectomy.
Methods
From October 2014 to December 2015, eight cases of VATS SL were performed in our institution by a single-port approach. The cohort included seven male patients and one female patient, with a median age of 56.0 years (range, 38–63 years). All patients were diagnosed with NSCLC by preoperative video bronchoscopic biopsy. An enhanced chest computed tomography scan was performed to confirm the tumor location, which was in the right upper lobe in four patients, right lower lobe in one patient, and left upper lobe in three patients. Enhanced brain magnetic resonance imaging, abdominal and cervical ultrasonography, and whole-body bone emission computed tomography were routinely performed to exclude metastases. Indications for single-port VATS SL were similar to those for conventional multi-port VATS, as follows: (I) central-type NSCLC involving lobar bronchus or part of the main bronchus; (II) tumor maximum diameter less than 6 cm; (III) exclusion of mediastinal lymph node and distant metastasis; (IV) patients’ lung function able to tolerate lobectomy or even pneumonectomy. Systematic lymph node dissection was conducted in all cases.
Surgical technique
Under general anesthesia with a double-lumen endotracheal tube, the patient was placed in a lateral position, and the surgeon stood at the patient’s ventral side (Figure 1). The unique incision was made at the 4th intercostal space on the anterior axillary line with no rib spreading. The incision was covered by a silicon wound protector, and the camera was inserted. The entire procedure was completed via monitor visualization.
Thoracoscopic exploration was conducted, and no extensive pleural adhesion was found in any patient. The inferior pulmonary ligament was mobilized first, after which the mediastinal pleura was opened from posterior to anterior. For right upper, right lower, and left upper SL, the hilar structure was dissected in order of pulmonary artery, pulmonary vein, bronchus. The pulmonary vein and the larger pulmonary arterial branch were divided with the stapler while the smaller pulmonary arterial branch was ligated by #7 silk thread. Hypoplastic interlobar fissure was divided with a stapler.
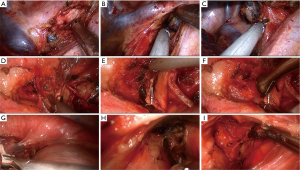
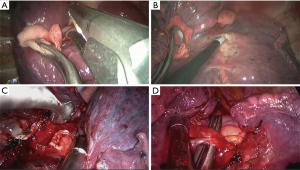
Next, systematic mediastinal lymph node dissection was performed (stations 2, 3, 4, 7, 8, and 9 for right pulmonary operations and stations 4, 5, 6, 7, 8 and 9 for left pulmonary operations). For right upper SL, we routinely divide the azygos vein to maintain sufficient exposure of the operating field, while for left upper SL, we lifted the trunk of the left pulmonary artery by threading a 1-0 silk suture through the posterior chest wall with a crochet needle to prevent visual interference and facilitate the procedure (13). For station 2R lymphadenectomy, the mediastinal pleura are opened from the triangular region delineated by the azygos vein arch, superior vena cava, and vagal nerve. Next, paratracheal lymph nodes around the azygos vein arch are dissected, the pleura inferior to the azygos vein arch is separated, and the vein is lifted to complete the lymph node dissection (Figure 2A-C). For station 4L lymphadenectomy, the vagal nerve trunk is exposed anteriorly and the structures in the aortopulmonary window space are mobilized. Next, we lift the mediastinal pleura and expose the recurrent laryngeal nerve, and the lymph node and surrounding fat are dissected between the aortic arch and pulmonary artery trunk (Figure 2D-F). The remaining left lower paratracheal lymph nodes are dissected posteriorly. Station 7L lymph nodes are the most difficult challenge because of their deep location. We retract the pulmonary artery and pericardium anteriorly to open the posterior mediastinal pleura. To obtain better exposure, we rotate the operating bed anteriorly to maneuver the patient into a semi-prone position. The esophagus is then retracted posteriorly, and the subcarinal lymph nodes are dissected (Figure 2G-I).
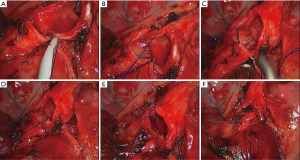
The bronchus was transected by scalpel and scissors. After the margins of the bronchus were confirmed as negative by intraoperative frozen-section examination, an end-to-end bronchial anastomosis was performed with continuous suturing using a single 3-0 prolene suture. A modified anastomosis technique developed by the author (Chen’s technique) was applied to the bronchial anastomosis. First, we made a stitch on the posterior bronchial wall and a knot outside the lumen, then the posterior quarter of the circumference was continuously sutured. The other three quarters of the circumference were sutured in sequence in the same way. The procedure was completed with a final knot outside the lumen (Figure 3).
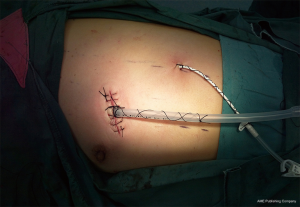
A thin-fiber bronchoscopic check was performed to observe the anastomosis. We then performed a leakage test and found no leakage at an airway pressure of 30 cmH2O (2.94 kPa). Finally, a 28 F drainage tube was placed at the incision, and a central venous catheter was placed in the seventh or eighth intercostal space on the posterior axillary line (Figure 4).
Results
All of the operations were completed uneventfully, without the need for conversion to conventional multi-port VATS or thoracotomy. There was no perioperative mortality. Major statistics, reported as medians or percentages, were as follows: operative duration, 234.5 min (range, 185–345 min); bronchial anastomosis duration, 38.0 min (30–43 min); blood loss, 65.0 mL (50–200 mL); number of lymph node dissected, 22.5 [18–37]. The postoperative complication rate was 37.5% (three out of eight patients), including two pulmonary infections and one atrial fibrillation. All patients recovered and were discharged uneventfully with symptomatic therapy. Pathological typing determined seven cases of squamous cell carcinoma and one case of adenocarcinoma. TNM staging of NSCLC revealed that two patients were in stage IB, 3 in stage IIA, 1 in stage IIB, and 2 in stage IIIA. The median follow-up was 7.5 months (2–15 months). No tumor recurrence or bronchial anastomotic complication was reported.
Discussion
In recent years, indications for single-port VATS were widely extended, complex pulmonary resection such as SL (14), double-sleeve resection (15), carinal resection (16) had been proved to be technically feasible. Although single-port VATS obviates the posterior and middle ports, there are notable differences in operative vision and surgical approach. The vision is directed at the target tissue, which according to Gonzalez-Rivas (17) mimics the open procedure. Although the field of vision from a single incision is limited, the rotation of a 30° camera provides a panoramic view (18). The surgical approach is also proximate to open surgery with respect to the absence of an optical plane created by conventional three-port triangulation, as Gonzalez-Rivas mentions (17). Another advantage of single-port VATS is the incision cosmesis, which is to some extent crucial for young patients. The ability to perform operations with one incision while following the oncological principles of major pulmonary resections also represents real progress. Two propensity-matched studies conducted by Shen et al. (19) and Wang et al. (20) showed that single-port VATS could achieve comparable or better perioperative outcomes than a multiple-incision approach. That said, these inspired reports still require larger sample sizes and randomized controlled studies for validation.
In terms of the operative time, bronchial anastomosis time, blood loss, and postoperative outcomes, the present study compares well with multi-port VATS SL literature (6,8,9). The success in performing single-port SL results from experience accumulated in three-port VATS lobectomy. Judging from our preliminary results (21) the learning curve of single-port lobectomy should be no longer than multi-port VATS for skilled surgeon, with the regression line for operative time falling to the average time of conventional surgeries after fewer than 30 procedures.
Mutual interference among the instruments is one of the main problems leading to prolonged operative time of single-port SL. To avoid this disadvantage and ensure a sufficient field of vision, we summarize our experience from three aspects. First, reasonable design of the incision. The incision should be somewhat distant from the lesion and hilar structure. In most cases, the incision is usually located at the 5th intercostal space, while for upper SL or in a heavier patient, the 4th intercostal space will be more feasible (12). Second, the surgeon should judiciously use both hands and choose an appropriate instrument. In our practice, for a right-handed surgeon, a harmonic scalpel, electrical hook, or other conventional straight instrument should be kept in the right hand, while the left hand holds prolonged and curved instruments, such as curved forceps and suction tools, to expose the targeted structures (Figure 5). Differences in the length of instruments and the space between them facilitate intraoperative procedures and ensure ideal vision. Third, close collaboration of the camera holder. An experienced camera holder is crucial for successful single-port VATS. In some reports, the surgeon and camera holder stand at the same side in front of the patient (10,19,20). In our institution, the camera holder routinely stands at the back of the patient, because we have found that staying at the same side can limit the movement of the surgeon and result in discomfort during the procedure. This step requires the camera holder to adapt a reverse-operational approach when performing subcarinal lymph node dissection, inferior pulmonary ligament separation, and other procedures near the diaphragm. The camera holder should keep the camera at the posterior edge of the incision and parallel to the surgeon’s instruments to prevent interference. Furthermore, the camera needs to be held by one hand on the base of the camera and the other hand near the incision to maintain steady vision (Figure 6).
Bronchial anastomosis is the most complex procedure in SL. A modified method developed by the author, historically applied in three-port VATS bronchial anastomosis (13), was introduced to our single-port approach. We summarize the characteristics of this technique as follows. First, the whole process of anastomosis was finished with continuous suture, which is considered time saving and technically simple owing to the tying of fewer knots (22). Moreover, continuous suturing reduced suture twisting, which is paramount in single-port VATS where visual angle is limited. Second, the deeply located and poorly exposed posterior wall was sutured first to reduce the tension at both ends, after which the anterior wall was sutured with the posterior wall visualized on the monitor. Good exposure of the posterior bronchial wall ensures the safety of the procedure. Third, proper adjustment of anastomosis tension was performed twice during the procedure. Deft suturing with 3-0 prolene can facilitate effective regulation of anastomosis tension and avoid anastomotic complications. In the present study, no anastomotic complications were observed up to the last follow-up, which proved the safety of this technique and potentially broadens its application range.
In conclusion, with accumulation of experience and improvements in instruments and surgical procedure in single-port VATS, it is feasible and safe to perform SL and systematic ML using a uniportal VATS approach. Application of Chen’s technique in bronchial anastomosis is easy and reliable, and leads to a satisfactory short-term clinical outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by ethics committee of Fujian Medical University Fujian Union Hospital (No. 2014029) and written informed consent was obtained from all patients.
References
- Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [Crossref] [PubMed]
- Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg 2003;76:1782-8. [Crossref] [PubMed]
- Maurizi G, D'Andrilli A, Anile M, et al. Sleeve lobectomy compared with pneumonectomy after induction therapy for non-small-cell lung cancer. J Thorac Oncol 2013;8:637-43. [Crossref] [PubMed]
- Hanna JM, Berry MF, D'Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5 Suppl 3:S182-9. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Mahtabifard A, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [Crossref] [PubMed]
- Nakanishi K. Video-assisted thoracic surgery lobectomy with bronchoplasty for lung cancer: initial experience and techniques. Ann Thorac Surg 2007;84:191-5. [Crossref] [PubMed]
- Li Y, Wang J. Video-assisted thoracoscopic surgery sleeve lobectomy with bronchoplasty: an improved operative technique. Eur J Cardiothorac Surg 2013;44:1108-12. [Crossref] [PubMed]
- Agasthian T. Initial experience with video-assisted thoracoscopic bronchoplasty. Eur J Cardiothorac Surg 2013;44:616-23. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Left lower sleeve lobectomy by uniportal video-assisted thoracoscopic approach. Interact Cardiovasc Thorac Surg 2014;18:237-9. [Crossref] [PubMed]
- Zhu Y, Xu G, Zheng B, et al. Single-port video-assisted thoracoscopic surgery lung resection: experiences in Fujian Medical University Union Hospital. J Thorac Dis 2015;7:1241-51. [PubMed]
- Xu G, Zheng W, Guo Z, et al. Complete video-assisted thoracoscopic surgery upper left bronchial sleeve lobectomy. J Thorac Dis 2013;5 Suppl 3:S298-300. [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic sleeve lobectomy and other complex resections. J Thorac Dis 2014;6:S674-81. [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:E2. [PubMed]
- Lyscov A, Obukhova T, Ryabova V, et al. Double-sleeve and carinal resections using the uniportal VATS technique: a single centre experience. J Thorac Dis 2016;8:S235-41. [PubMed]
- Gonzalez-Rivas D. VATS lobectomy: surgical evolution from conventional VATS to uniportal approach. ScientificWorldJournal 2012;2012:780842.
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Shen Y, Wang H, Feng M, et al. Single- versus multiple-port thoracoscopic lobectomy for lung cancer: a propensity-matched study†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i48-53. [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [Crossref] [PubMed]
- Cheng K, Zheng B, Zhang S, et al. Feasibility and learning curve of uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2016;8:S229-34. [PubMed]
- Nakagawa T, Chiba N, Ueda Y, et al. Clinical experience of sleeve lobectomy with bronchoplasty using a continuous absorbable barbed suture. Gen Thorac Cardiovasc Surg 2015;63:640-3. [Crossref] [PubMed]


