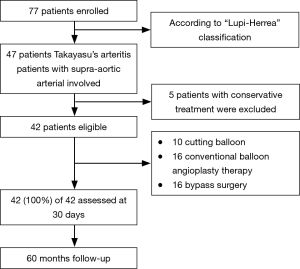
Outcomes of different treatments on Takayasu’s arteritis
Introduction
Takayasu’s arteritis (TA) is a nonspecific chronic inflammation of the aorta and its branches. The frequency of supra-aortic arterial (SAA) lesions, including innominate, subclavian, carotid, and vertebral, in TA patients, was 31% to 45% (1-3). Mwipatayi, et al reported that 17% of TA patients suffered from transient ischemic attack (TIA) or stroke, which resulted in 9.5% mortality (4). Furthermore, 50% patients needed surgical treatment for TA disease and most patients underwent reintervention (5). The efficacy of surgical treatment in patients with TA has not been established.
Surgical bypass is considered as the efficient therapy for TA patients with symptomatic, irreversible SAA lesions; however, the early and late complications after bypass surgery, such as postoperative bleeding, cerebrovascular accidents, anastomotic aneurysm were the primary causes of mortality (6,7). Furthermore, previous reports of endovascular treatment of SAA lesions in TA patients were limited by the endovascular instruments such as conventional balloon, the frequency of active disease at the time of intervention, the type of endovascular technique used (e.g., use of a stent), and the differences in the post-procedural medical treatment (8-10).
Cutting balloon angioplasty proved to be safe and efficient in the treatment of graft-to-vein anastomotic stenosis and non-arteriosclerotic renal artery stenosis, with significantly higher assisted primary patency than that of the conventional balloon angioplasty (8-10). Gumus et al. reported that cutting balloon angioplasty was an efficient surgical treatment to treat renal artery stenosis due to TA, however, there is a lack of clinical studies about the cutting balloon angioplasty in the treatment of SAA lesion involvement in TA patients (11).
In this study, we compared the endovascular treatment including cutting balloon angioplasty and conventional balloon angioplasty, along with the surgical bypass in TA patients with SAA lesions.
Methods
Patients
Between January 2010 to March 2015, forty-two patients (32.88±10.28 years), including seven males and thirty-five females, with SAA lesions underwent surgical treatment in our institution. The diagnosis of TA was confirmed by the presence of three or more criteria of The American College of Rheumatology (12). Preoperative clinical characteristics, surgical therapy manners of cutting balloon, conventional angioplasty and bypass surgery, primary and secondary patency, re-intervention, as well as early (<30 days) and late complications, were reviewed retrospectively. Blood pressure difference between arms >10 mmHg in systolic blood pressure was defined as clinic signs of left subclavian artery stenosis or occlusion. Percent stenosis, binary open or closed was measured on CT before surgery and repeated confirm during surgery by angiographic. Stenosis percentage was compared with proximal part diameter. Stenosis type was classified by the length of the stenotic lesion, focal (<2 cm) and diffuse (≥2 cm). Most diffuse lesion patients were treated by bypass surgery, whereas the focal lesion was first treated with the conventional angioplasty and its inefficiency resolved by the cutting balloon (Flow chart: Figure 1). The study complied with the Helsinki Declaration and was approved by the local ethical committee.
Procedure
Patients that underwent operation in the inactive phase were assessed by erythrocyte sedimentation rate (ESR, <15 mm/h in men, <20 mm/h in women) and C-reactive protein (CRP, <0.9 mg/dL). All endovascular procedures were performed under the control of anticoagulation; heparin was administered at 70 IU/kg and the activated coagulation time was controlled above 200 s during the procedure. A 6 Fr sheath and guiding catheter (RDC-I) were inserted from the common femoral artery. The size of the balloon was decided by the angiographic measurement of the reference diameter as follows. In the case of a non-ostial lesion, the ostium diameter was determined as a reference. In the case of an ostial lesion, the portion distal to the lesion or the contralateral artery was measured. In the event of post-stenotic dilatation, we also measured the distal portion of the dilated site, especially in the patient with the bilateral artery. The conventional balloon sized identically to the reference diameter was chosen for the initial treatment. It was inflated from within to assess the rate of rated burst pressure, and not inflated further when the patient complained of a headache.
For cutting balloon angioplasty, we selected a cutting balloon similar or smaller in size than the reference diameter and never used an oversized equipment. When the lesion was narrow, an undersized cutting balloon was used initially to avoid destruction of the blade due to the bending discrepancies between the stenosis and the normal vessel. Only one size of cutting balloon was used for either of the procedure. The cutting balloon, which was not advanced through the lesion, was retracted in the sheath. All cutting balloons were inflated to the minimum pressure at which the balloon waist disappeared, to minimize the vessel injury. The inflation time of the cutting balloon was about 30s. After deflation, the cutting balloon was carefully removed, and the absence of blade destruction was confirmed immediately. The lesion diameter was also assessed after the inflation. If the balloon diameter was still smaller than the reference diameter, or the stenosis percentage >70%, the lesion was defined as insufficiently dilated, and the cutting balloon angioplasty was either repeated, or the conventional angioplasty was added, unless the patient complained. The cutting balloon angioplasty procedure and effect was shown in Figure 2. Stent was used in both insufficiently dilated endovascular treatment.
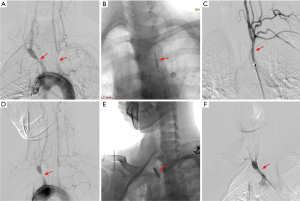
Sixteen TA patients with SAA lesions were underwent bypass surgical procedure, from ascending aorta to carotid artery bypass. The bypass conduit was externally supported by polytetrafluoroethylene grafts in fifteen patients and autogenous saphenous vein in one.
Follow-up protocol
All patients were clinically followed up with outpatient. Every patient was requested to have a repeat computed tomography angiography (CTA) at 3, 6, 12 months in the first postoperative year, and then annually thereafter until the fifth years or the end of this study in March 2015.
Analysis and statistics
Categorical variables are reported as number (present) and continuous variables as mean (SD) or median (25th to the 75th interquartile range), depending on variable distribution. Group comparisons were analyzed with the Student’s t-test or Wilcoxon rank-sum test for numeric variables and the chi-square or Fisher exact test for categorical variables. All analyses were performed using Empower (R) (www.empowerstats.com, X&Y solutions, Inc., Boston, MA, USA) and R (http://R-project.org). Significance was attributed at P<0.05. Cumulative patency rates of the grafts were estimated by Kaplan-Meier analysis. The life-table method was used to analyze the effects of various factors on the primary patency rate.
Results
Fundamental clinical characteristics of TA patients
Demographic and disease characteristics were summarized in Table 1. All the patients underwent surgical intervention for the inactive disease stage as assessed by ESR <20 mm/h and CRP <0.9 mg/dL. Twenty patients were administered with anti-inflammatory medicines in the perioperative time. Mean age was 29.6 years in cutting balloon angioplasty, 36.37 years in conventional balloon angioplasty, and 31.44 years in bypass surgery group. A significant difference was not observed. Four patients exhibited stroke history before the operation, two from the conventional balloon angioplasty group, one in cutting balloon angioplasty group, and one in bypass surgery group. Pulselessness (24/42, 57.1%: cutting balloon angioplasty 5, conventional balloon angioplasty 12, bypass surgery 7) and dizziness (18/42, 42.9%: cutting balloon angioplasty 5, conventional balloon angioplasty 6, bypass surgery 7) were no significant difference. Thirty-eight common carotid arteries (right 19, left 19), 19 vertebral arteries (right 12, left 7), and 24 left subclavian arteries were affected. Stenosis rate was not markedly different between the groups, 88.0%±4.21% in cutting balloon angioplasty, 85.6%±4.78% in conventional balloon angioplasty, and 88.1%±3.59% in bypass surgery. Eleven patients with diffuse artery lesions underwent bypass surgery, and 17 patients with focal lesions were subjected to endovascular therapy.
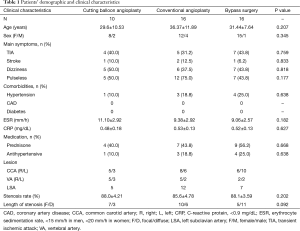
Full table
Perioperative period results
Perioperative period results were shown in Table 2. Thirteen bare-metal stents (self-expanding) were planted in 12 patients in the conventional balloon angioplasty group, but none was used in the cutting balloon angioplasty group. The cutting balloon angioplasty procedure consumed the least time (47.3±6.7 min) as compared to the conventional angioplasty (80.1±22.5 min) and the bypass surgery (304.8±46.9 min, P<0.01). The percutaneous transluminal angioplasty of two patients was unsuccessful because the guidewire could not pass the lesions, and thus, they were converted to bypass surgery. Arterial dissection occurred in 1patient.
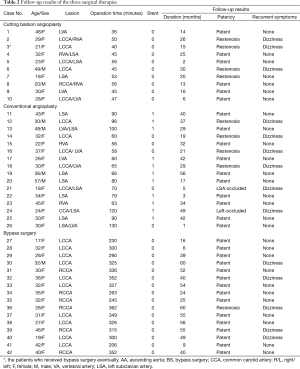
Full table
Follow-up results
The mean follow-up time was 30.07±17.96 months (range, 1–60 months, presented in Table 2). All the patients’ symptoms were alleviated or improved after the surgical procedure; the dizziness without image restenosis occurred in four patients, while in one with anastomotic stenosis in bypass surgery (5, 31.2%). Blood pressure difference between arms was relieved in both the endovascular treatment groups owing to the left subclavian artery blood flow restoration. Restenosis in four patients was confirmed by CTA in the cutting balloon angioplasty group (3, 30%), among which one had no symptoms. In the conventional balloon angioplasty group, five artery restenosis or occlusions and one stent occlusion were found (6, 37.5%). There was no significant difference between cutting balloon angioplasty group and conventional balloon angioplasty group. The rate of restenosis in the bypass surgery group (1, 6%) was lower than both endovascular treatment (P<0.05).
Cumulative patency rates were shown in Figure 3. Primary patency rates of the cutting balloon angioplasty group at 3, 6, and 24 months were 100%, 100%, and 54.54%, respectively. The same was 95%, 78.23%, and 58.29% of the conventional balloon angioplasty group at 3, 6, and 24 months, respectively, while it was 100% and 90.9% for bypass surgery group at 3 and 36 months. The rate of primary patency in the bypass surgery group was higher than cutting balloon angioplasty and conventional balloon angioplasty group at 24 months (P<0.05).
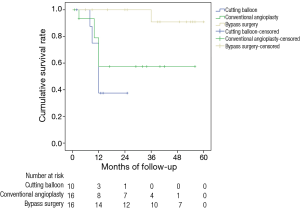
Short-term complications
Short-term complications (<30 days) and late complications were shown in Table 3. Cerebral hyperperfusion syndrome with intracerebral hemorrhage occurred in one patient. This patient demonstrated left common carotid and left subclavian artery diffused stenosis and received ascending aorta-left common carotid artery bypass. Subsequently, she recovered without any left-over disability. Any cardiac accidents and bradycardia with hemodynamic instability did not occur in the TA patients.
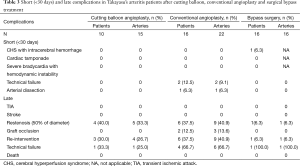
Full table
Midterm to long-term complications
None of the patients reported either TIA or stroke during the follow-up duration. Restenosis or occlusion developed in nine out of 22 arteries (40.9%) in conventional angioplasty, compared with one of the 16 arteries (6.3%) after surgical bypass (P=0.018). The differences of restenosis or occlusion rate between cutting balloon angioplasty group and conventional balloon angioplasty group were not significant (P=0.738). The re-intervention results were presented in Table 3. Three patients (4 arteries) in cutting balloon angioplasty group and six patients (9 arteries) in conventional balloon angioplasty group required re-intervention. Among them, threeout of four (75%) treated by cutting balloon angioplasty were patent as compared to the three out of nine arteries (33.3%) dealt with by conventional angioplasty that was patent (P=0.266). No mortality was observed in this cohort.
Discussion
Conventional balloon angioplasty treatment and bypass surgery treating symptomatic TA patients were previously reported (7,13). On the other hand, there is a lack of evidence on the efficiency of cutting balloon angioplasty in SAA lesions of TA patients.
In the present study, SAA arteries reconstructed by surgical bypass showed better patency than those treated by cutting balloon angioplasty. Because of the TA arteries injury is a vessel-specific pattern in the response of the various arterial beds to inflammatory stimuli (14), stenosis artery replaced by surgical bypass is more effective alleviate arterial inflammatory stimuli and restenosis than endovascular treatment. However, a substantial distinctness in the symptom recurrence rate between the surgical bypass group and cutting balloon angioplasty group was not observed. These findings were similar to those by AbuRahma et al. in a study of atherosclerotic occlusive disease in a subclavian artery (15). They reported that the surgical bypass showed better long-term patency than endovascular intervention, but with increased perioperative complications.
The primary patency between the cutting balloon angioplasty and conventional balloon angioplasty was not significantly altered. It was reported that PTA with stent implantation results exceeded the SAA lesions of the TA patients (6,7,13). However, the feasibility of stenting for SAA lesions of Takayasu disease has not been established (9). In addition, patients with Takayasu disease are usually young, and the durability of the stents for these patients is yet controversial (16). The risk of undergoing a surgical intervention increased during the first phase of the disease (particularly in the first 16 months from the onset) and likely reached a plateau from 6 years (5). Inflammatory lesions in TA are mostly generated in the activated endothelial cells (17). The Cutting balloon was designed to alleviate vascular trauma and thereby reduce neointimal hyperplasia (8) to restore stenotic arteries and improve long-term patency. Therefore, we chose to use a cutting balloon and avoided stent implantation. Moreover, cutting balloon angioplasty was reported to improve the patency rate in treating in-stenting restenosis lesions. According to the results of this study, cutting balloon angioplasty appeared to be advantageous over conventional angioplasty in SAA lesions of TA.
The procedure would carry the risk of vessel rupture if the cutting balloon was inflated by extremely high pressure. So the PTA should be conducted very carefully (11). Our result showed cutting balloon angioplasty to be advantageous for solid lesions of TA. However, its efficacy for primary patency was controversial. Further examination in follow-up phase is necessary to assess the patency, especially for cases with stent implantation and consolidated lesions.
Limitation
The results of our research might be limited by the relative small number of subjects. Thus more high-quality and large-sample randomized study are required to further verify our study. In addition, the long-term patency rate of cutting ballon therapy is still controversial. It still needs longer follow-up.
Conclusions
This study has shown that cutting balloon angioplasty may be a safe and effective procedure for SAA lesions in TA patients, in particular for youths. Cutting balloon angioplasty shows promising short-term results and can be considered as a less-invasive alternative for non-diffused lesions. However, endovascular treatment is associated with a higher risk of long-term restenosis or occlusion than bypass surgery, which has a better patency rate.
Acknowledgements
Funding: This study was financed by the National Natural Science Foundation of China (81330034 and 81273522), and the outstanding youth medical talent program of Shanghai (XYQ2013087).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committee of Changhai Hospital. The Institutional Review Board number is CHEC-2016-67.
References
- Park MC, Lee SW, Park YB, et al. Clinical characteristics and outcomes of Takayasu's arteritis: analysis of 108 patients using standardized criteria for diagnosis, activity assessment, and angiographic classification. Scand J Rheumatol 2005;34:284-92. [Crossref] [PubMed]
- Moriwaki R, Noda M, Yajima M, et al. Clinical manifestations of Takayasu arteritis in India and Japan--new classification of angiographic findings. Angiology 1997;48:369-79. [PubMed]
- Hong C, Zeng T, Zhao J, et al. Takayasu's arteritis misdiagnosed as mediastinal malignant lymphoma: a case report and review of the literature. J Thorac Dis 2014;6:E115-9. [PubMed]
- Mwipatayi BP, Jeffery PC, Beningfield SJ, et al. Takayasu arteritis: clinical features and management: report of 272 cases. ANZ J Surg 2005;75:110-7. [Crossref] [PubMed]
- Vanoli M, Daina E, Salvarani C, et al. Takayasu's arteritis: A study of 104 Italian patients. Arthritis Rheum 2005;53:100-7. [Crossref] [PubMed]
- Miyata T. The Asia Pacific meeting for vasculitis and ANCA workshop 2012: surgical treatment for Takayasu's arteritis. Clin Exp Nephrol 2014;18:296-300. [Crossref] [PubMed]
- Kim YW, Kim DI, Park YJ, et al. Surgical bypass vs endovascular treatment for patients with supra-aortic arterial occlusive disease due to Takayasu arteritis. J Vasc Surg 2012;55:693-700. [Crossref] [PubMed]
- Saleh HM, Gabr AK, Tawfik MM, et al. Prospective, randomized study of cutting balloon angioplasty versus conventional balloon angioplasty for the treatment of hemodialysis access stenoses. J Vasc Surg 2014;60:735-40. [Crossref] [PubMed]
- Tanaka R, Higashi M, Naito H. Angioplasty for non-arteriosclerotic renal artery stenosis: the efficacy of cutting balloon angioplasty versus conventional angioplasty. Cardiovasc Intervent Radiol 2007;30:601-6. [Crossref] [PubMed]
- Hu J, Huang H, Zhang X, et al. Stent placement for treatment of long segment (≥80 mm) carotid artery stenosis in patients with Takayasu disease. J Vasc Interv Radiol 2012;23:1473-7. [Crossref] [PubMed]
- Gumus B, Cevik H, Vuran C, et al. Cutting balloon angioplasty of bilateral renal artery stenosis due to Takayasu arteritis in a 5-year-old child with midterm follow-up. Cardiovasc Intervent Radiol 2010;33:394-7. [Crossref] [PubMed]
- Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129-34. [Crossref] [PubMed]
- Kim HJ, Lee CS, Kim JS, et al. Outcomes after endovascular treatment of symptomatic patients with Takayasu's arteritis. Interv Neuroradiol 2011;17:252-60. [PubMed]
- Pryshchep O, Ma-Krupa W, Younge BR, et al. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation 2008;118:1276-84. [Crossref] [PubMed]
- AbuRahma AF, Bates MC, Stone PA, et al. Angioplasty and stenting versus carotid-subclavian bypass for the treatment of isolated subclavian artery disease. J Endovasc Ther 2007;14:698-704. [Crossref] [PubMed]
- Schmidt J, Kermani TA, Bacani AK, et al. Diagnostic features, treatment, and outcomes of Takayasu arteritis in a US cohort of 126 patients. Mayo Clin Proc 2013;88:822-30. [Crossref] [PubMed]
- Arnaud L, Haroche J, Mathian A, et al. Pathogenesis of Takayasu's arteritis: a 2011 update. Autoimmun Rev 2011;11:61-7. [Crossref] [PubMed]