Annual or biennial lung cancer CT screening?
Annual low-dose computed tomography (LDCT) screening has been found to be an efficient lung cancer screening program for a high-risk population (1). According to the United States guidelines annual screening is recommended for high-risk individuals, defined as those aged 55–74 years, with a 30 pack-year smoking history and who currently smoke or have quit within the past 15 years. This is based on the results of the National Lung Screening Trial (NLST), which provided an insight into how to decrease death due to lung cancer (1). NLST was a randomized trial comparing annual screening by LDCT with chest radiograph for three years in 53.454 high-risk persons at 33 United States medical centers. The trial showed that lung cancer screening with LDCT can reduce lung cancer mortality by 20% compared to chest X-ray for current or former heavy smokers (1).
In Europe, the results of the ongoing Dutch-Belgian lung cancer screening trial (NELSON) are awaited, in order to issue screening guidelines. In total seven randomized trials of LDCT screening remain in progress in Europe (2). The intergroup for thoracic oncology and French-speaking oncology [the French Intergroup (IFCT) and the Groupe d’Oncologie de Langue Française (GOLF)], recommend that individuals aged 55–74 years and have a 30-pack-year smoking history to be screened with LDCT, after being informed about the risks and benefits of screening (3). The Cancer Care Ontario Programme (CCOP) issued guidelines in 2013 targeting the same group of high-risk individuals but suggesting biennial screening after two consecutive years of negative scanning (4). Before a decision about screening implementation is made in Europe, there are several issues that need to be addressed (5).
In the Patz et al. retrospective study, published in The Lancet Oncology, it was demonstrated that annual screening may be unnecessary for individuals who have a negative low-dose CT prevalence screen (T0) (6). A negative T0 is defined as no non-calcified nodules greater than 4 mm in diameter and no other suspicious findings. A retrospective cohort analysis of data from the NLST showed that lung cancer incidence and mortality are significantly lower in participants with negative T0 screen in comparison with those with positive T0 screen. The yield of lung cancer at the first annual screen (T1) among high-risk participants with a negative T0 screen was 0.34%, a third of that reported for the T0 screen in all T0-screened participants (1.0%) (6). Less than one per 1,000 participants (0.09%) was diagnosed with lung cancer between the T0 and T1 screens among the participants with a negative T0 screen.
Indeed several groups have tried to answer questions such as whether annual screening can keep the balance of health-care costs, harms and benefits of the patients or whether a biennial screening interval should be considered for individuals who have a negative prevalence screen, using data from ongoing clinical trials (7-9).
Data from the NELSON trial suggest that a screening interval of at least 2 years can be considered in individuals who have no pulmonary nodules on screens one and two, done one year apart. The NELSON study focuses on the in-depth analysis of CT scans using volumetric analysis. A volume cutoff of 27 mm3 or greater had a sensitivity exceeding 95% for the detection of lung cancer (8). Of interest is that the 2-year lung cancer probability for participants without any pulmonary nodules on screens one and two was 0.4% compared to 2.5% for those with CT-detected nodules. For the first time, Edward Patz and colleagues demonstrated in the dataset of a completed trial the benefit of an extended screening interval for individuals with a negative prevalence screen (6). These results make clear the scope for further defining a low-risk group of individuals by using data from their initial screen results (Figure 1).
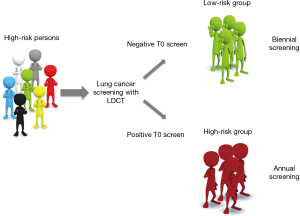
At least in Europe we still have the opportunity to consider who needs annual screening and who can have LDCT in more extended intervals, before lung cancer screening is embedded into a national policy. Decisions regarding implementation of a lung cancer-screening program should be based upon several factors, including the cost-effectiveness analysis of a screening program, psychosocial harms as well as long-term accumulation of radiation exposure. According to the NLST trial, the cost of screening per life saved is unknown but likely to be high, given the almost 95% false-positive rate leading to the need for additional studies and ongoing screening, and the relatively low absolute number of deaths prevented (1). There are several other challenges in lung cancer screening programs as was described by Abbie Begnaud at ASCO 2016. In the screening program at the University of Minnesota Health, the orders for screening were fewer than had been expected, possibly because of uncertainty about insurance coverage. But even when exams were ordered, only 63% of them were completed. This problem was attributed, in part, to reimbursement uncertainty and out-of-pocket payment for the exam. In addition, patient anxiety may have prevented many patients from scheduling exams, even if they had information about the need to do so.
Other modalities apart from LDCT are investigated for lung cancer screening (10). For instance, monitoring “sentinel” circulating tumor cell (CTC)-positive chronic obstructive pulmonary disease (COPD) patients may allow early diagnosis of lung cancer. Ilie et al. found that the annual screening of five CTC-positive COPD patients by LDCT detected lung nodules 1–4 years after CTC detection, leading to prompt surgical resection and histopathological diagnosis of early-stage lung cancer (11). Other than blood potential biosamples for biomarker analysis include airway epithelium, sputum and exhaled breath (10). To this scope, a biospecimen repository of serially collected blood, sputum, and urine samples has been established from participants of the NLST, for future investigation. Finally, it is needless to comment on the great relevance of integrating smoking cessation practices into future national lung cancer LDCT screening programs.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial/Commentary/Perspective commissioned by the Section Editor Long Jiang (Second Affiliated Hospital, Institute of Respiratory Diseases, Zhejiang University School of Medicine, Hangzhou, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Field JK, van Klaveren R, Pedersen JH, et al. European randomized lung cancer screening trials: Post NLST. J Surg Oncol 2013;108:280-6. [Crossref] [PubMed]
- Couraud S, Cortot AB, Greillier L, et al. From randomized trials to the clinic: is it time to implement individual lung-cancer screening in clinical practice? A multidisciplinary statement from French experts on behalf of the French intergroup (IFCT) and the groupe d'Oncologie de langue francaise (GOLF). Ann Oncol 2013;24:586-97. [Crossref] [PubMed]
- Roberts H, Walker-Dilks C, Sivjee K, et al. Screening high-risk populations for lung cancer: guideline recommendations. J Thorac Oncol 2013;8:1232-7. [Crossref] [PubMed]
- Field JK, Oudkerk M, Pedersen JH, et al. Prospects for population screening and diagnosis of lung cancer. Lancet 2013;382:732-41. [Crossref] [PubMed]
- Patz EF Jr, Greco E, Gatsonis C, et al. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol 2016;17:590-9. [Crossref] [PubMed]
- Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308-15. [Crossref] [PubMed]
- Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332-41. [Crossref] [PubMed]
- Duffy SW, Field JK, Allgood PC, et al. Translation of research results to simple estimates of the likely effect of a lung cancer screening programme in the United Kingdom. Br J Cancer 2014;110:1834-40. [Crossref] [PubMed]
- Hensing TA, Salgia R. Molecular biomarkers for future screening of lung cancer. J Surg Oncol 2013;108:327-33. [Crossref] [PubMed]
- Ilie M, Hofman V, Long-Mira E, et al. "Sentinel" circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One 2014;9:e111597. [Crossref] [PubMed]


